Abstract
Abstract 1479
Despite a functioning immune system, malignant tumors develop strategies to avoid immune recognition and elimination by T cells. Gene therapy can be used to render T cells capable of targeting tumors in an antigen-specific manner. The ability to genetically manipulate a T cell ex vivo provides new opportunities to enhance their biological activity in vivo. To explore new treatment approaches we propose a novel paradigm in which T cells are developed as biological delivery vehicles for desired genes and nanoparticles. We have combined gene therapy with nanotechnology to generate T cells that express a chimeric antigen receptor (CAR) selectively expressed on T cells operating within the tumor microenvironment and to introduce radiolabeled gold nanoparticles for tracking T cells in vivo.
T cells can home to tumor microenvironments to exert their immunoreceptor-dependent cytolytic activity. However, despite the ability of T-cell receptors (TCRs) and CAR to recognize tumor cells, the anti-tumor effect can be incomplete. The ability to transfer a wide range of materials into T cells via electroporation can be exploited to adapt T cells as biological delivery agents capable of targeting specific tissues. This method may also circumvent the limited biodistribution of “raw” nanomaterials due to rapid clearance by the liver and other reticuloendothelial system (RES) organs. Gold is an attractive biocompatible nanomaterial which can be synthesized in compliance with current good manufacturing practice (cGMP) guidelines required for clinical-grade production and can be chemically functionalized for specific imaging or therapeutic functions.
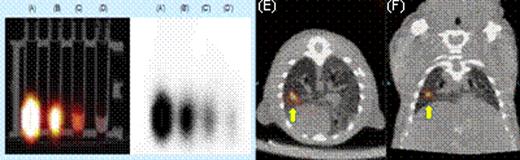
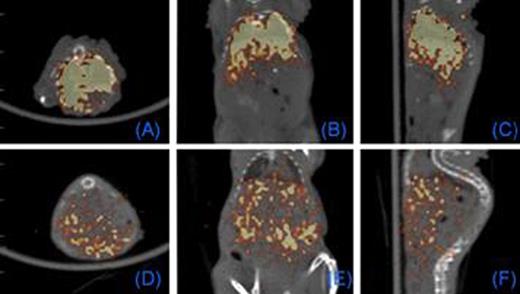
We first tested whether commercially available nanoparticles can be electro-transferred into cultured and primary human T cells. 43 nm diameter latex nanoparticles and 7 nm gold nanoparticles (GNPs) were electro-transferred into T cells and visualized by TEM and confocal imaging (Figure 1). We have modified GNPs on the surface with the chelator, diethylenetriaminepentaacetic acid (DTPA), for stable coordination with 111In followed by GNP PEGylation. We have shown that these radiolabeled GNPs can be readily electro-transferred into T cells suitable for combined single-photon emission computed tomography (SPECT) and computed tomography (CT) (Figure 2). We used a clinical SPECT/CT scanner to detect 111In-GNP in T cells (∼2.1 × 104 nanoparticles/cell) at a sensitivity of ∼760 cells/mL. After developing electroporation protocols of nanoparticles and in vivo imaging of 111In-GNPs, additional sensitivity was achieved by modifying the chelating chemistry using the macrocyclic chelator, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), to bind 64Cu to GNPs, increasing the number of gold particles/cell, and using 64Cu-labeled GNPs for imaging by positron emission tomography (PET). Before tail vein injection to a mouse, 11.4 mCi was detected from 10 million T cells (suspended in 300 μL PBS) electroporated using a BTX ECM830 device with the following settings: 1 kV/cm, 4 ms duration, single square pulse (Figure 3). The estimated concentration of nanoparticles transferred into T cells was ∼2.3 × 105 nanoparticles/cell as determined by a gamma counter (2470 Wizard, PerkinElmer) and nanoparticle titration. While 20 nm GNPs were used for 111In labeling, 7 nm GNPs were chosen for 64Cu labeling because of the improved (10-fold) electroporation efficiency.
We are currently investigating whether multi-functional GNPs encapsulating chemotherapy drugs can add both in vivo T-cell imaging capability and enhanced cytotoxicity to CAR-redirected T cells. In aggregate, this may improve the potency of clinical-grade genetically modified T cells as a vehicle with the imaging capability for targeted delivery of drug-loaded GNPs to the tumor microenvironment.
(A) 43 nm size latex nanoparticles confocal microscope (Red: nanoparticles, Blue: DAPI stained nuclei) (B) 7 nm GNPs inside a T cell (JEOL 2010 TEM).
(A) 43 nm size latex nanoparticles confocal microscope (Red: nanoparticles, Blue: DAPI stained nuclei) (B) 7 nm GNPs inside a T cell (JEOL 2010 TEM).
SPECT Imaging. (A) through (D) show the radioactivity of electro-transferred 111In-GNPs into T cells. (E) axial and (F) coronal views of directly injection of T cells containing111In-GNPs.
SPECT Imaging. (A) through (D) show the radioactivity of electro-transferred 111In-GNPs into T cells. (E) axial and (F) coronal views of directly injection of T cells containing111In-GNPs.
PET Imaging of T cells loaded with intracellular 64Cu-GNPs. 0.5 (A-C) and 18 (D-F) hours after tail vein injection of T cells. Each panel: axial, coronal, sagittal views, respectively.
PET Imaging of T cells loaded with intracellular 64Cu-GNPs. 0.5 (A-C) and 18 (D-F) hours after tail vein injection of T cells. Each panel: axial, coronal, sagittal views, respectively.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.