Abstract
Abstract 1856
Sixty to 80% of patients (pts) with higher-risk myelodysplastic syndromes (MDS) require RBC transfusions to manage symptoms of anemia. Transfusion-associated complications include iron overload, transmitted infections, alloimmunization, and decreased overall survival (OS) (Cancer 2006;106:2087; JCO 2005;23:7594). The phase 3 AZA-001 study compared azacitidine (AZA) with conventional care regimens (CCR) in pts with higher-risk MDS (Lancet Oncol 2009;10:223). For all pts randomized to AZA, the reported median number of RBC transfusion events in the 56-day pre-randomization baseline (BL) period was relatively low (1.0, range 0 – 10), since 68/179 (38%) AZA-treated pts required no RBC transfusions (Haematologica 2009:Abstract 0813). However, pretreatment transfusion burden in the 111 RBC transfusion-dependent (TD) pts treated with AZA was substantial in many cases. It is unknown to what extent pts with higher RBC transfusion requirements at BL achieve transfusion independence (TI) and derive an OS benefit from AZA treatment.
To assess OS in pts with higher-risk MDS treated with AZA in the AZA-001 study who were RBC TD at BL, based on pretreatment transfusion needs and posttreatment transfusion status.
In AZA-001, 179 pts with higher-risk MDS (FAB-defined RAEB, RAEB-t, or CMML with >10% blasts, and IPSS risk Int-2 or High) were randomized to AZA (75 mg/m2/d SC × 7d q 28d). Of these pts, 111 (62%) were RBC TD at BL and 50 pts (45%) achieved TI during AZA treatment (Lancet Oncol, 2009;10:223). (Because so few pts who received CCR became TI on treatment [13/114, 11%], this analysis included only AZA-treated pts.) Erythropoietic stimulating agents were not permitted on study. BL RBC TD was defined as receiving ≥1 transfusion during the 56-day pre-randomization BL period, and per IWG 2000 criteria, TI on treatment required an RBC transfusion-free period ≥56 consecutive days. BL characteristics of pts who remained TD and of pts who achieved TI were evaluated and compared descriptively. Median OS was estimated using Kaplan-Meier (KM) methods for pts with pretreatment RBC transfusion burden of ≥1 (ie, all pts in the analysis), 1, or ≥2 transfusions (similar to IWG 2006 criteria for TD). The KM OS distributions for the on-treatment TI and TD groups were compared within in each cohort defined by pre-treatment RBC transfusion burden using the log-rank test.
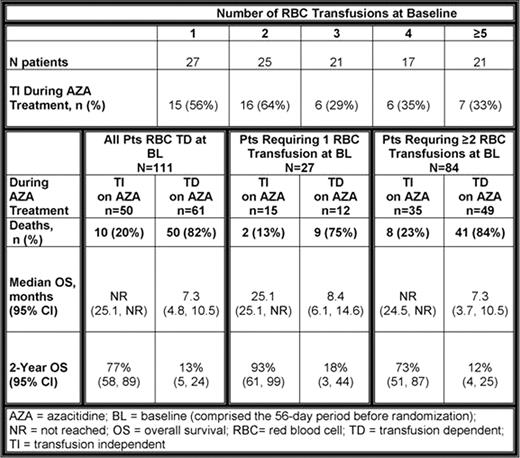
Among the 111 AZA-treated pts who were RBC TD at BL, median age was 70 yrs (range 42 – 83), 77% were male, and 91% had ECOG performance scores of 0–1. Most pts (84/111, 76%) required ≥2 RBC transfusions in the 56-day pre-randomization BL period (Table). Median number of RBC transfusions at BL was 2 (range 1 – 10) in pts who achieved TI, and 3 (range 1 – 9) in pts who remained TD. Of pts who required ≥2 RBC transfusions at BL, 35 (42%) achieved TI. One-third (7/21) of pts with the highest RBC transfusion requirements at BL (≥5 transfusions) achieved TI during AZA treatment. Median OS was improved 3-fold for all cohorts of pts who achieved RBC TI with AZA, regardless of RBC transfusion burden at BL, compared with median OS in pts who remained TD (log-rank p<0.0001, all comparisons). Similarly, OS at 2 years in pts who achieved RBC TI during AZA treatment was 60–75% greater than OS at 2 years in pts who remained TD (Table). Of pts with the highest RBC transfusion needs at BL, 73% who achieved TI were alive at 2 years compared with only 12% of pts who remained TD. Generally, pts who remained TD on AZA were more likely to have WHO AML (20-30% blasts), higher LDH, and require platelet transfusions at BL than pts who achieved TI. However, pts who achieved TI on AZA more often had IPSS poor cytogenetics and 3 cytopenias at BL than pts who remained TD.
A substantial proportion of pts with higher-risk MDS who required ≥2 RBC transfusions pretreatment achieved TI with AZA. TI in this analysis was associated with a marked improvement in OS.
Seymour:Celgene: Honoraria, Speakers Bureau. Santini:Celgene: Honoraria. Fenaux:Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Merck: Honoraria; Cephalon: Honoraria; Novartis: Honoraria; J&J: Honoraria. Giagounidis:Celgene: Consultancy, Honoraria. Sanz:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Finelli:Celgene: Consultancy. Lucy:Celgene: Employment, Equity Ownership. Backstrom:Celgene: Employment, Equity Ownership. Beach:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.