Abstract
Abstract 2082
Stem cell transplantation (SCT) is the only available cure for patients with thalassemia major (TM). Transfusional iron overload is a common complication with SCT. Data defining the natural history of iron overload, its impact on morbidity and mortality, and its treatment in transplant recipients is limited. There are currently no randomized trials addressing post SCT iron removal in children with TM. This prospective randomized trial will compare the efficacy, safety, and convenience of phlebotomy versus deferasirox for the treatment of iron overload in children with TM who have undergone allogeneic SCT.
Chelation naïve patients between 2 – <20 years of age with a serum ferritin (SF) level ≥500 ng/ml on at least 2 monthly occasions, and a liver iron concentration (LIC) >3 mg Fe/g dry liver weight (dw) as determined by R2 MRI will be randomized to two groups: phlebotomy or deferasirox. The starting dose of deferasirox will be 10 mg/kg/day adjusted every 3 months in increments of 5 mg/kg to a maximum dose of 20 mg/kg/day according to monthly SF and its trend. For phlebotomy, 6 ml/kg of blood will be withdrawn every 2 weeks. The amount of blood removed during each phlebotomy session will be recorded, and the hemoglobin and iron concentrations will be calculated. Correlation between total body iron stores (TBISs) and LIC will be undertaken. Patients will undergo monthly SF, quarterly serum/urine iron, and yearly cardiac echo, liver R2 and cardiac T2* MRI measurement. Continuous adverse event monitoring will be made. Total liver iron content will be determined by R2 MRI and liver volume measurement. Quality of life (QoL) will be measured by the PEDSQL questionnaire. Treatment duration will be one year but interrupted if SF drops below 300 ng/ml and/or with major adverse events. We herein describe baseline characteristics of 27 chelation naïve, hepatitits C and B negative children with TM (16 males, 11 females) cured by SCT from an HLA-identical family member (unrelated donor in 1 patient) who are enrolled in the above trial.
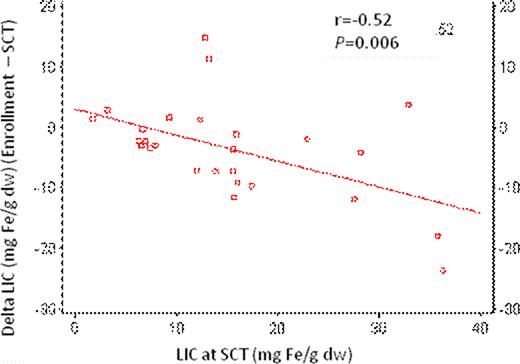
Patients’ characteristics at enrollment are outlined in Table 1 . Ten patients (37.0%) had SF <1000 ng/ml, 11 (40.8%) had SF 1000–2500 ng/ml, while 6 (22.2%) had SF >2500 ng/ml. Twelve patients (44.4%) had LIC 3-<7 mg Fe/g dw, 8 (29.6%) had LIC 7–15 mg Fe/g dw, while 7 (26.0%) had LIC >15 mg Fe/g dw. Five (18.5%) patients had cardiac T2* <20 msec. Overall, 9 patients (33.3%) had significant iron overload defined as SF >2500 ng/ml, or LIC >15 mg Fe/g dw, or T2* <20 msec. On multivariate regression, predictors of change in SF at enrollment relative to transplant were: SF and LIC (P<0.05); whereas predictors of change in LIC at enrollment relative to transplant were: SF, LIC, and total number of transfusions (TNT) (P<0.05). The change in LIC correlated negatively with LIC at SCT (Pearson's correlation, r=-0.52; P=0.006). A negative change in LIC was observed with an LIC at SCT of 10 mg Fe/g dw and above (Figure 1). There were no statistically significant correlations between cardiac T2* and any of LIC, SF, or TNT; or between LIC and any of age, risk, class, or gender.
This report sheds light on the natural history of post transplant iron overload in children cured of TM and underscores the fact that such children continue to have iron overload for years post treatment. It also highlights correlations between SF, LIC, and TNTs in this setting. As the trial continues enrolling more patients, it is expected to generate important data comparing 2 modalities of iron overload treatment (deferasirox vs. phlebotomy) in this patient population.
Patients’ characteristics at enrollment.
| Parameter . | Median . | Range . |
|---|---|---|
| Age (years) | 12.48 | 3.12–19.62 |
| Follow up post SCT (years) | 3.1 | 1.2–4.6 |
| Hemoglobin (g/dl) | 12.2 | 9.9–15.9 |
| Total number of transfusions (n) | 88 | 25–330 |
| SF (ng/ml) | 1144.3 | 502.5–5884 |
| Liver volume (ml) | 1251.2 | 553.8–2111 |
| LIC (mg Fe/g dw) | 8.3 | 3.1–36.6 |
| Cardiac T2* (msec) | 27.8 | 8.5–41.5 |
| Ejection fraction (%) | 68 | 58–75 |
| Serum iron (ìg/dl) | 131.2 | 43.5–307.5 |
| Total iron binding capacity (ìg/dl) | 246.5 | 189.5–336.2 |
| Transferrin (%) | 1.79 | 1.09–2.47 |
| Alanine aminotransferase(IU/l) | 24 | 18.9–78 |
| Aspartate aminotransferase (IU/l) | 24.4 | 9.9–214.5 |
| Blood urea nitrogen (mg/dl) | 13.5 | 6.9–22 |
| Creatinine (mg/dl) | 0.45 | 0.27–0.68 |
| Creatinine clearance (ìM/l) | 123 | 55.9–545 |
| Microalbuminuria (mg/24 hrs) | 10.1 | 3.5–403.5 |
| Parameter . | Median . | Range . |
|---|---|---|
| Age (years) | 12.48 | 3.12–19.62 |
| Follow up post SCT (years) | 3.1 | 1.2–4.6 |
| Hemoglobin (g/dl) | 12.2 | 9.9–15.9 |
| Total number of transfusions (n) | 88 | 25–330 |
| SF (ng/ml) | 1144.3 | 502.5–5884 |
| Liver volume (ml) | 1251.2 | 553.8–2111 |
| LIC (mg Fe/g dw) | 8.3 | 3.1–36.6 |
| Cardiac T2* (msec) | 27.8 | 8.5–41.5 |
| Ejection fraction (%) | 68 | 58–75 |
| Serum iron (ìg/dl) | 131.2 | 43.5–307.5 |
| Total iron binding capacity (ìg/dl) | 246.5 | 189.5–336.2 |
| Transferrin (%) | 1.79 | 1.09–2.47 |
| Alanine aminotransferase(IU/l) | 24 | 18.9–78 |
| Aspartate aminotransferase (IU/l) | 24.4 | 9.9–214.5 |
| Blood urea nitrogen (mg/dl) | 13.5 | 6.9–22 |
| Creatinine (mg/dl) | 0.45 | 0.27–0.68 |
| Creatinine clearance (ìM/l) | 123 | 55.9–545 |
| Microalbuminuria (mg/24 hrs) | 10.1 | 3.5–403.5 |
Change in LIC versus LIC at SCT.
Inati:Novartis Pharmaceuticals: Speakers Bureau. St Pierre:Resonance Health: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Taher:Novartis Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.