Abstract
Abstract 2905
Myelodysplastic syndromes (MDS) are a group of malignant bone marrow stem cell disorders characterized by a predisposition for transformation to acute myelogeneous leukemia (AML). MDS disorders are heterogeneous in their morphology, cytogenetics, survival time, and ability to transform to AML. Although numerous classification schemes have been developed to provide a reproducible method for estimating patient survival and risk of leukemic evolution, research continues to identify factors that affect prognosis and survival time (e.g., treatment type, WHO FAB subgroups, karyotype, and transfusion status) and refine existing classification schemes. The goal of this analysis was to evaluate the effect of platelet counts on survival and disease progression to AML among thrombocytopenic (platelet count <100 × 109/L) MDS patients (n = 303) using the International MDS Risk Analysis Workshop (IMRAW) database.
The IMRAW dataset includes demographic, clinical and prognostic information for patients treated in the United States, Europe, and Japan who were diagnosed with MDS between 1972 and 1994. Platelet count (× 109/L) was categorized as follows: 0 to <20, 20 to <30, 30 to <50, 50 to <70, 70 to <80, 80 to <90, 90 to <100, and ≥100. Survival was modeled using the Kaplan-Meier (K-M) estimator. One-, two-, and three-year survival rates and median, quartile, and restricted mean survival time were estimated using K-M methods. Time to evolution to AML was modeled using the Nelson-Aalen (N-A) cumulative hazard estimator with death prior to onset of AML as a competing risk event. One-, two-, and three-year cumulative rates of AML evolution were generated using N-A methods. Mean, median, and quartile values for time to AML evolution were also summarized. Cox proportional hazards modeling was used to estimate the hazard ratio (HR) for survival and evolution of MDS to AML.
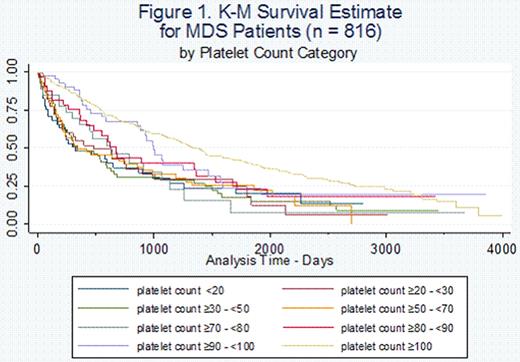
Among thrombocytopenic MDS patients (n = 303), one-, two- and three-year survival were 58%, 44%, and 33%, respectively. Median survival was 19.6 months, with the lowest among those with a platelet count 0 to <20 × 109/L (10.9 months) and the highest among those with a platelet count 90 to <100 × 109/L (33.2 months). Patients with platelet counts (x 109/L) <70, 70 to <90, and ≥90 formed three distinct survival groups for approximately three years after MDS diagnosis (Figure 1). Across platelet categories, one-, two- and three-year survival ranged from 48.2% to 83.4%, 37.3% to 68.0%, and 30.5% to 39.9%, respectively (Table 1). Among non-thrombocytopenic MDS patients (platelet count ≥100 × 109/L), the one-, two- and three-year survival were 83.1%, 68.6%, and 59.1%, respectively, and the median survival was 47.3 months. Results of Cox models for thrombocytopenic MDS patients suggested an increased risk of death with platelet counts <90 × 109/L compared to platelet counts 90 to <100 × 109/L and no relationship was shown between strength of the HR and decreasing platelet count when adjusted for gender, age, year of diagnosis and institution (Table 1). Among thrombocytopenic MDS patients who developed AML (n = 73), median time to AML diagnosis from MDS diagnosis was 9.2 months. One-, two- and three-year evolution to AML rates were 21%, 30%, and 38%, respectively.
Platelet count in thrombocytopenic MDS patients may be clinically relevant to survival and should be evaluated further. Platelet count does not appear to be associated with risk of evolution to AML. Evaluation of these relationships in a larger sample is warranted.
1-, 2-, and 3-year survival among thrombocytopenic MDS patients (n = 303) by platelet count
| . | Survival . | ||||
|---|---|---|---|---|---|
| . | 1 Year (%) . | 2 Year (%) . | 3-Year (%) . | Median (months) . | Adjusted HR (95% CI) . |
| Platelet Count (× 109/L) | |||||
| 0–<20 | 48.2 | 37.3 | 30.5 | 10.9 | 1.60 (0.94–2.73) |
| ≥20–<30 | 54.8 | 42.7 | 29.6 | 16.0 | 1.63 (0.96–2.76) |
| ≥30–<50 | 48.3 | 31.0 | 29.1 | 11.5 | 1.91 (1.18–3.09) |
| ≥50–<70 | 49.6 | 41.7 | 33.0 | 11.1 | 1.51 (0.93–2.48) |
| ≥70–<80 | 65.4 | 46.1 | 28.8 | 20.9 | 1.67 (0.92–3.01) |
| ≥80–<90 | 68.5 | 43.5 | 39.9 | 22.5 | 1.45 (0.83–2.53) |
| ≥90–<100 | 83.4 | 68.0 | 38.9 | 33.2 | – |
| . | Survival . | ||||
|---|---|---|---|---|---|
| . | 1 Year (%) . | 2 Year (%) . | 3-Year (%) . | Median (months) . | Adjusted HR (95% CI) . |
| Platelet Count (× 109/L) | |||||
| 0–<20 | 48.2 | 37.3 | 30.5 | 10.9 | 1.60 (0.94–2.73) |
| ≥20–<30 | 54.8 | 42.7 | 29.6 | 16.0 | 1.63 (0.96–2.76) |
| ≥30–<50 | 48.3 | 31.0 | 29.1 | 11.5 | 1.91 (1.18–3.09) |
| ≥50–<70 | 49.6 | 41.7 | 33.0 | 11.1 | 1.51 (0.93–2.48) |
| ≥70–<80 | 65.4 | 46.1 | 28.8 | 20.9 | 1.67 (0.92–3.01) |
| ≥80–<90 | 68.5 | 43.5 | 39.9 | 22.5 | 1.45 (0.83–2.53) |
| ≥90–<100 | 83.4 | 68.0 | 38.9 | 33.2 | – |
Yong:Amgen Inc: Employment, Equity Ownership. Kuehn:Amgen Inc: Research Funding. Kelsh:Amgen Inc: Research Funding. Wagner:Amgen Inc: Research Funding. Yang:Amgen Inc: Employment, Equity Ownership. Franklin:Amgen Inc: Employment, Equity Ownership.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.