Abstract
Abstract 3557
Autologous stem cell transplantation for patients with amyloidosis results in high reported hematologic and organ response rates compared with conventional chemotherapy.
Four-hundred and thirty-four patients have undergone transplantation between March 8, 1996, and April 13, 2010 Table 1. Clinical parameters seen in patients with amyloid are given in Table 2. The overall day-100 mortality seen in this group of patients is 44 of 434 patients (10%).
Characteristics of Patients with Amyloidosis Prior to Autologous Stem Cell Transplant (n=434)
| . | Number . | Percentage (%) . |
|---|---|---|
| Sex M/F | 259/175 | 60 |
| Renal involvement | 304 | 70 |
| Cardiac involvement | 214 | 49 |
| Peripheral nerve involvement | 54 | 12 |
| Liver involvement | 59 | 14 |
| Autonomic involvement | 15 | 3 |
| CTS | 54 | 12 |
| Tongue enlargement | 44 | 10 |
| Number organs involved: | ||
| 1 | 203 | 47 |
| 2 | 168 | 39 |
| >2 | 63 | 14 |
| . | Number . | Percentage (%) . |
|---|---|---|
| Sex M/F | 259/175 | 60 |
| Renal involvement | 304 | 70 |
| Cardiac involvement | 214 | 49 |
| Peripheral nerve involvement | 54 | 12 |
| Liver involvement | 59 | 14 |
| Autonomic involvement | 15 | 3 |
| CTS | 54 | 12 |
| Tongue enlargement | 44 | 10 |
| Number organs involved: | ||
| 1 | 203 | 47 |
| 2 | 168 | 39 |
| >2 | 63 | 14 |
Laboratory Values in Patients with Amyloidosis (n=434)
| . | Units . | Median . | Interquartile Range (IQR) . |
|---|---|---|---|
| Liver edge N=59 | cm BCM | 7 | (4–10) |
| Albumin | g/dL | 2.7 | (1.9–3.3) |
| Creatinine | mg/dL | 1.1 | (0.9–1.3) |
| Cholesterol | mg/dL | 241 | (185–336) |
| Alkaline phosphatase | U/L | 88 | (70–129) |
| β2 Microglobulin | mg/L | 2.59 | (2.08–3.04) |
| 24-hour urine total protein | g/day | 3.68 | (0.28–7.45) |
| % Marrow PC | 7 | (3–13) | |
| IVS septal thickness | mm | 12 | (10–14) |
| EF | % | 65 | (60–69) |
| Age | years | 57 | (51–63) |
| Months from diagnosis to SCT | 4 | (3–7) |
| . | Units . | Median . | Interquartile Range (IQR) . |
|---|---|---|---|
| Liver edge N=59 | cm BCM | 7 | (4–10) |
| Albumin | g/dL | 2.7 | (1.9–3.3) |
| Creatinine | mg/dL | 1.1 | (0.9–1.3) |
| Cholesterol | mg/dL | 241 | (185–336) |
| Alkaline phosphatase | U/L | 88 | (70–129) |
| β2 Microglobulin | mg/L | 2.59 | (2.08–3.04) |
| 24-hour urine total protein | g/day | 3.68 | (0.28–7.45) |
| % Marrow PC | 7 | (3–13) | |
| IVS septal thickness | mm | 12 | (10–14) |
| EF | % | 65 | (60–69) |
| Age | years | 57 | (51–63) |
| Months from diagnosis to SCT | 4 | (3–7) |
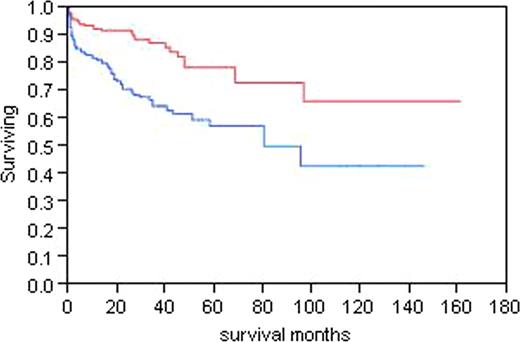
The most important determinant of outcome is stage defined by BNP and troponin levels. Figure 1 demonstrates the survival for patients for all 3 cardiac stages. Troponin <.035; NT-proBNP <332. Stage 1 both low, Stage 3 both elevated. The survival of all patients based on whether they achieved a complete response, a partial response (>50%), or no response was analyzed. Median survival has not been reached for the complete response group, is 107 months for the partial response group, and is 32 months for the no response group (p<0.0001). Figure 2, divides the patients at the median dFLC level of 13.5 mg/dL demonstrating that for patients with a higher level of free light chain, the median survival is 87.6 months and has not been reached for those with a lower level of free light chain. A proportional hazards model for variables that impact on survival was done in all patients, and the only relevant predictor of outcome was stage (p<0.0001). When the same analysis was performed using a landmark of 6 months, the only predictor of outcome was stage (p=0.0005). When best response was incorporated into the landmark model, it was the strongest predictor of survival. The amyloid stage predicted survival (p= 0.0005), and the best response to therapy (p <0.0001). When the same analysis was performed in the entire group of 434, stage and best response each remained significant (p<0.0001).
Survival by the pretreatment d FLC greater than or less than13.5 mg/dL. Top line <13.5, bottom >=13.5
Survival by the pretreatment d FLC greater than or less than13.5 mg/dL. Top line <13.5, bottom >=13.5
There is a high response rate associated with high-dose therapy that was not observed in the melphalan and prednisone era. For eligible patients who can be transplanted safely, high-dose melphalan is an effective therapy for many patients with amyloidosis. Best response and stage at diagnosis are the best predictors of overall survival.
Lacy:Celgene: Research Funding. Dispenzieri:Celgene: Honoraria, Research Funding; Binding Site: Honoraria. Kumar:Celgene: Consultancy, Research Funding; Millennium: Research Funding; Merck: Consultancy, Research Funding; Novartis: Research Funding; Genzyme: Consultancy, Research Funding; Cephalon: Research Funding.
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract