Abstract
Abstract 4559
Patients with acute myeloid leukemia (AML) and t(8;21) generally have a favorable prognosis with a higher rate of first complete remission (CR1) and higher rate of cure with high-dose cytarabine (HDARA-C). However, some studies comparing stem cell transplantation (SCT) and conventional consolidation chemotherapy raised questions regarding improved survival with autologous SCT (ASCT) in these patients. Moreover, there has been some concern that the rate of relapse after HDARA-C is higher than previously reported and resultingly, only 50 – 60% of these patients are cured with contemporary treatment. Role of reduced intensity conditioning (RIC)-SCT has not been determined in this population. To date, few studies have analyzed the outcome of RIC-SCT for this population specifically. From this point of view, we performed a prospective phase II study to evaluate the efficacy of RIC-SCT for AML with t(8;21) and also compared the results with those of the historical cohort who treated with ASCT or myeloablative conditioning (MC)-SCT in our institution.
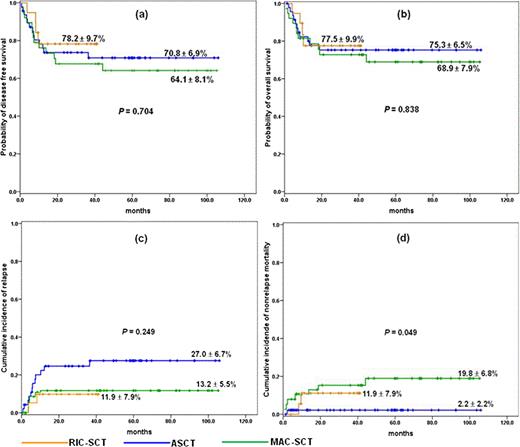
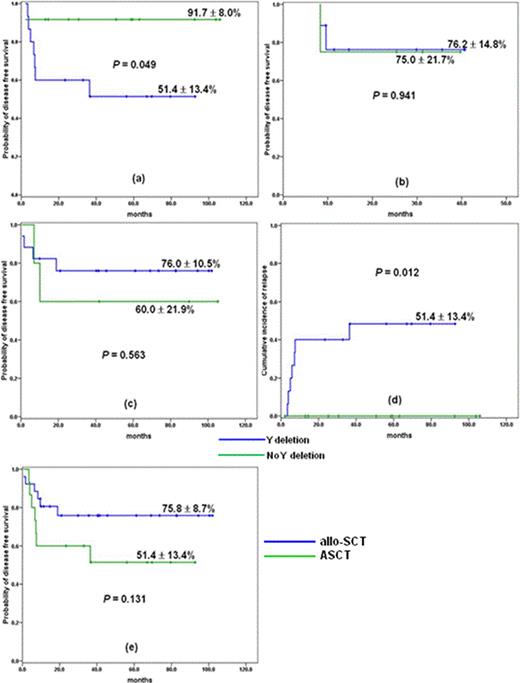
We included adult patients aged between 16 and 65 with AML who had t(8;21) with or without additional aberration at the time of diagnosis. Since Mar 2007, we transplanted 19 consecutive adult patients with AML and t(8;21) after RIC from matched sibling (n = 12) or unrelated (n = 7) donor. Patients in RIC-SCT group were given a RIC regimen consisting of fludarabine (30 mg/m2/d, days –6, –5, –4, –3, and –2), busulfan (3.2 mg/kg/d, days –6 and –5) and low dose total body irradiation (400 cGy). Their clinical features were compared with historical patients who were transplanted with autografting (n = 47) or MC (n = 38) in our institution from 2001. The pretransplant characteristics among the three groups were well balanced except for patient age and time to SCT. For recipients of RIC, probability of overall survival (OS) and disease free survival (DFS) was 77.5 ± 9.9% and 78.2 ± 9.7%, respectively, with a median follow-up of 30 months (range: 9.5+ – 41.2+ months) for surviving patients (Fig 1a and b). 2-year cumulative incidence of relapse (CIR) and nonrelapse mortality (NRM) was 11.9 ± 7.9%, respectively (Fig. 1c and d). OS and DFS in this group were not different from those of ASCT and MC-SCT group. CIR appears to be lower than that of ASCT, but failing to demonstrate statistical difference. Patients in ASCT group showed clearly lower NRM (P = .049). By univariate analysis, age (£ 50 vs > 50 years) seems to be a dominant factor affecting DFS and OS in RIC-SCT and ASCT group (P = 0.25 and 0.29, respectively, for RIC-SCT group, and P = 0.36 and 0.13, respectively, for ASCT group). In ASCT group, presence of –Y was associated with inferior DFS (p = 0.049, Fig. 2a), whereas this difference disappeared when RIC or MC was applied for these patients (Fig. 2b and c). CIR was significantly lower in patients without –Y than with –Y (P = 0.012, Fig. 2d), while there was no difference in NRM between the two subgroups. Of 41 male patients with –Y, probability of DFS of allogeneic SCT recipients was slightly better than that of ASCT, although failing to demonstrate a statistical difference (Fig. 2e).
Overall transplant outcome. probability of (a) DFS, and (b) OS, (c) cumulative incidence of relapse, (d) cumulative incidence of NRM
Overall transplant outcome. probability of (a) DFS, and (b) OS, (c) cumulative incidence of relapse, (d) cumulative incidence of NRM
Outcome according to –Y status. (a) DFS of ASCT group (b) DFS of RIC-SCT group, (c) DFS of MC-SCT group, (d) CIR by –Y status (e) DFS according to transplant modality
Outcome according to –Y status. (a) DFS of ASCT group (b) DFS of RIC-SCT group, (c) DFS of MC-SCT group, (d) CIR by –Y status (e) DFS according to transplant modality
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.