Complement is important for both innate and adaptive immunity, providing the first line of defense against invading micro-organisms.2,,–5 Complement is also involved in the phagocytosis of apoptotic cells and cellular debris in response to injury,3,5 the regulated removal of neurons during brain development,6 neuroprotection, neuronal migration, organ regeneration, and homing of hematopoietic stem cells.2 As with coagulation, complement activation involves proteolytic activation of multiple factors under the tight regulation of inhibitors,3,5 and, not surprisingly, regulatory disturbances can result in disease. During acute inflammation, complement anaphylatoxins mediate changes in vascular flow, permeability, leukocyte extravasation, and migration, sometimes contributing to tissue damage after ischemia and reperfusion.7,8 Autoantibody-mediated complement activation and inherited or acquired alterations of complement components can result in a variety of conditions, including systemic lupus erythematosus, rheumatoid arthritis, paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis type II (dense deposit disease), and age-related macular degeneration (AMD).2,3,5,7 AMD is a major cause of vision loss in the elderly when immune deposits (drusen) containing complement form between the retinal pigment epithelium and the underlying choroid. Atrophy and neovascularization of the subretinal tissue gradually leads to loss of central vision. Polymorphisms and deletions of genes that mostly affect the complement alternative pathway (CFH, CFB, C3) are major risk factors for AMD, suggesting that dysregulation of complement leads to the progression of AMD.2,5
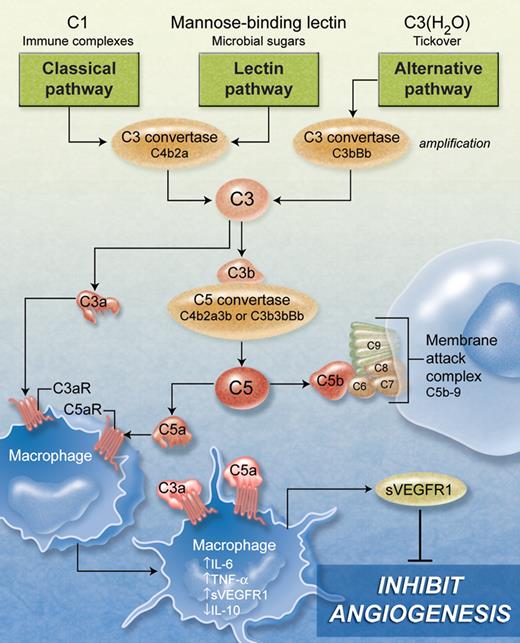
The complement system involves more than 40 soluble and surface-bound proteins, with membrane-associated receptors and regulators interacting with effectors to target microbes or damaged cells for elimination. Complement response is initiated via the classical, lectin, and alternative pathways, which converge on the proteolytic activation of C3 (see figure) to produce C3a and C3b. C3b contributes to the formation of the C5 convertase, which cleaves C5 to produce C5a and C5b. The effectors C3a and C5a (anaphylatoxins) bind to their respective receptors C3aR and C5aR (C5a also binds to C5a receptor-like 2, C5L2), found on neutrophils, eosinophils, mast cells, monocytes/macrophages, dendritic cells, endothelial cells, astrocytes, and microglia, as well as other tissues.9 C5b recruits C6, C7, C8, and C9 to form the terminal membrane attack complex, leading to pore formation and cell destruction.2,3,5
The study by Langer and colleagues in this issue reveals the importance of complement in regulating neovascularization in a mouse model of retinopathy of prematurity (ROP).1 Their ROP model used newborn mice exposed to high oxygen concentration (75%) in an incubator followed by exposure to room air, leading to retinal hypoxia causing a hypoxia-driven proangiogenic response with increased pathologic neovascularization. Retinal angiogenesis was quantified by enumerating stained epiretinal vascular cell nuclei. They found unexpectedly that neovascularization was increased in C3-deficient (C3−/−) mice compared with controls. The inhibitory role of the C5a receptor during angiogenesis was shown by increased neovascularization in C5aR−/− mice compared with control mice. Antibody-mediated blockade of C5 and terminal complement activation in wild-type mice also resulted in increased neovascularization. Similarly, wild-type mice injected with C5aR antagonist peptide showed increased neovascularization, whereas treatment with C5a agonist peptide reduced retina neovascularization, confirming the inhibitory role of C5a and C5aR in this process. Furthermore, treatment of C3-deficient mice with C3a and C5a reduced the enhanced neovascularization effect observed in C3−/− mice, suggesting that C3a and C5a are inhibitory effectors of pathologic hypoxia-driven retinal neovascularization.
Although no direct effect on angiogenesis was observed with C3a or C5a in endothelial cells, C5a-stimulated macrophages caused an inhibitory response. Specifically, C5a-treated macrophages had an antiangiogenic signature (increased IL-6, TNF-α, soluble VEGF receptor 1 [sVEGFR1], and decreased IL-10 mRNA). Secreted sVEGFR1 protein was increased in the supernatant of C5a-stimulated mouse macrophages and human monocytes, and the latter supernatant also inhibited angiogenesis in an in vitro Matrigel tube formation assay. Immunodepletion of sVEGFR1 from the supernatant reversed the inhibitory effect, confirming the key role of sVEGFR1 in monocyte/macrophage-mediated down-regulation of angiogenesis (see figure).
Complement activation and its potential effect on macrophage-mediated inhibition of angiogenesis. The proteolytic activation of C3 can be mediated by the classical, lectin, and alternative pathways, initiated by C1 complex, mannose-binding lectin, and H2O-bound C3, respectively. C3 produces C3a and C3b, which can participate in both the alternative pathway C3 convertase (amplification) and C5 convertase. C5 produces C5a and C5b, which can recruit C6, C7, C8, and C9 to form the terminal membrane attack complex causing pore formation and cell destruction. C3a and C5a anaphylotoxins bind to receptors (C3aR and C5aR) on monocytes/macrophages and many other cells causing an antiangiogenic response reflected by increased IL-6, TNF-α, sVEGFR1, and decreased IL-10 mRNA. Increased sVEGFR1 secretion from monocytes/macrophages inhibits hypoxia-driven retinal neovascularization and Matrigel angiogenesis. (Professional illustration by A. Y. Chen.)
Complement activation and its potential effect on macrophage-mediated inhibition of angiogenesis. The proteolytic activation of C3 can be mediated by the classical, lectin, and alternative pathways, initiated by C1 complex, mannose-binding lectin, and H2O-bound C3, respectively. C3 produces C3a and C3b, which can participate in both the alternative pathway C3 convertase (amplification) and C5 convertase. C5 produces C5a and C5b, which can recruit C6, C7, C8, and C9 to form the terminal membrane attack complex causing pore formation and cell destruction. C3a and C5a anaphylotoxins bind to receptors (C3aR and C5aR) on monocytes/macrophages and many other cells causing an antiangiogenic response reflected by increased IL-6, TNF-α, sVEGFR1, and decreased IL-10 mRNA. Increased sVEGFR1 secretion from monocytes/macrophages inhibits hypoxia-driven retinal neovascularization and Matrigel angiogenesis. (Professional illustration by A. Y. Chen.)
To address whether macrophages modulate angiogenesis in vivo, the authors depleted mouse macrophages using clodronate liposomes. Macrophage depletion in C3−/− mice reversed the enhanced neovascularization effect, confirming the requirement of macrophages for the complement-mediated antiangiogenic effect in ROP. The role of complement on in vivo angiogenesis was also studied using Matrigel plugs implanted into mice for 7 days. Matrigel angiogenesis in C3−/− and in antibody-blocked C5 or C5aR−/− mice was significantly increased compared with control mice. The enhanced neovascularization effect in C3−/− and C5aR−/− mice was lost after macrophage depletion, confirming that macrophages are key mediators in this process.
The antiangiogenic effect of complement during postnatal retinal neovascularization, which may affect other tissues as well, has hitherto not been appreciated. On the contrary, complement has been shown to have a stimulatory effect in choroidal neovascularization,10 and patients with polymorphisms in complement regulator genes have an increased risk of developing AMD.2,3,5 This discrepancy may be explained by the fact that the retinal pigment epithelium involved in AMD is not implicated in proliferative ROP. In addition, proangiogenic stimuli in AMD may overcome any inhibitory effects, suggesting the presence of distinct proliferative eye pathologies. The current studies imply that complement inhibitory drugs must be used with caution in AMD patients with concurrent microvascular disease such as diabetic retinopathy because such therapies may actually aggravate vascular proliferation in the eye.
These studies provide important new insights into the inhibitory role of complement during angiogenesis, yet some questions remain. For example, since C5a can also be generated in the absence of C3 by thrombin,11 what is the potential role of coagulation in complement-mediated regulation of angiogenesis? Are any of the many other cell types possessing C3a and C5a receptors involved in angiogenesis? What is the role of the C5L2 receptor in this process? The availability of gene knockout mice and studies of patients with complement defects will undoubtedly help address some of these questions. Importantly, the current studies shed new light on the role of the complement system during angiogenesis, which may also have implications for its role in tumor development.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■