Abstract
Progress in our understanding of iron-restricted erythropoiesis has been made possible by important advances in defining the molecular mechanisms of iron homeostasis. The detection and diagnostic classification of iron-restricted erythropoiesis can be a challenging process for the clinician. Newer assays for markers of inflammation may allow more targeted management of the anemia in these conditions. The availability of new intravenous iron preparations provides new options for the treatment of iron-restricted erythropoiesis. This review summarizes recent advances regarding the detection, evaluation, and management of iron-restricted erythropoiesis.
Introduction
Iron is an essential component of heme and hemoglobin, and therefore restriction of iron delivery to erythrocyte precursors can limit erythropoiesis. Progress in our understanding of iron-restricted erythropoiesis has been made possible by important advances in defining the molecular mechanisms of iron homeostasis. Iron restriction is the common mechanism in several clinical settings via 4 conditions: absolute iron deficiency, iron sequestration (impaired iron trafficking), functional iron deficiency (imbalance between the surging iron requirements of the stimulated erythroid marrow and iron availability), and hereditary conditions with impaired iron transport and utilization.1
Conditions that can be associated with iron-restricted erythropoiesis are summarized in Table 1. The detection and diagnostic classification of iron-restricted erythropoiesis can be a challenging process for the clinician. In the past, the diagnosis of iron deficiency anemia was seemingly straightforward, based on traditional biochemical markers such as serum iron, iron saturation, and ferritin from the chemistry laboratory. The anemia of chronic disease was considered a diagnosis of exclusion based on an evaluation excluding absolute iron deficiency and of other possible, known causes of anemia.
More physiologic markers for the evaluation of iron-restricted erythropoiesis are now available from the hematology laboratory, which can avoid the ambiguity of the traditional biochemical assays in the setting of inflammation.2 Newer assays for markers of inflammation may allow more targeted management of the anemia in these conditions.3
Finally, the availability of multiple intravenous iron preparations, some of which mitigate the risk of anaphylaxis, now provides alternatives for iron supplementation therapy for the treatment of iron-restricted erythropoiesis.4 This review summarizes recent advances regarding the detection, evaluation, and management of iron-restricted erythropoiesis.
Pathophysiology
Iron-restricted erythropoiesis can develop from a variety of causes. Absolute iron deficiency anemia can result from low dietary iron content, impaired iron absorption, or excessive iron loss (bleeding), but in these conditions, iron-regulatory and erythropoietic mechanisms remain functional. Anemia with functional iron deficiency develops during increased erythropoiesis mediated either by endogenous erythropoietin responses to anemia, or by therapy with erythropoiesis-stimulating agents (ESAs): the iron supply, though adequate for baseline erythropoiesis, cannot meet the erythron requirements of increased erythropoiesis.5 In this review, however, we will focus mostly on the pathophysiology of iron-sequestration anemia, in which the underlying mechanisms have recently been elucidated. Table 1 also highlights several other causes of iron-restricted anemia due to various molecular defects, including rare mutations in proteins involved in iron transport, recycling and utilization.
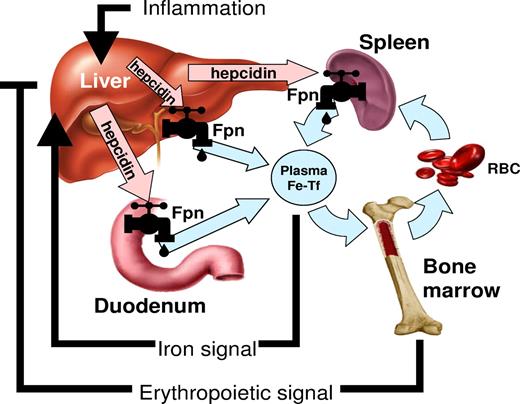
In the last 10 years, understanding of the regulation of iron homeostasis has changed substantially. A small peptide hormone, hepcidin, emerged as the central regulator of iron absorption, plasma iron levels, and iron distribution. Hepcidin is secreted by mainly by hepatocytes, and to a lesser extent by macrophages and adipocytes. The hormone acts by inhibiting iron flows into plasma from macrophages involved in recycling of senescent erythrocytes, duodenal enterocytes engaged in the absorption of dietary iron, and hepatocytes that store iron.6 Hepcidin exerts its activity by binding to the iron exporter ferroportin and causing its degradation. As ferroportin is the major entryway for iron into plasma, decrease in ferroportin reduces the iron available for erythropoiesis. Hepcidin production is regulated by iron and erythropoietic activity (Figure 1).6 Increased plasma and stored iron stimulate hepcidin production, which in turn inhibits dietary iron absorption. Hepcidin regulation by iron is predominantly transcriptional and appears to center on the bone morphogenetic protein (BMP) receptor, its signaling pathway and its accessory proteins,6 with BMP6 proposed as a key physiologic regulator of hepcidin. Plasma iron (as diferric transferrin) may be sensed by the 2 transferrin receptors TfR1 and TfR2 that convey the information to the BMP receptor complex via the accessory proteins HFE and hemojuvelin. Ablation of HFE, TfR2, hemojuvelin or BMP6, or liver-specific ablation of SMAD4, a key component of the BMP receptor signaling pathway, causes deficient and dysregulated hepcidin synthesis. It is not known how iron stores (as contrasted with extracellular or plasma iron) regulate hepcidin synthesis.
The role of hepcidin in iron metabolism. Hepcidin-ferroportin interaction determines the flow of iron into plasma. Hepcidin concentration is in turn regulated by iron, erythropoietic activity, and inflammation.6
The role of hepcidin in iron metabolism. Hepcidin-ferroportin interaction determines the flow of iron into plasma. Hepcidin concentration is in turn regulated by iron, erythropoietic activity, and inflammation.6
Iron deficiency and increased erythropoietic activity suppress hepcidin. Very low hepcidin concentrations are observed in patients with absolute iron-deficiency anemia, or anemias with high erythropoietic activity.3,7 Low hepcidin allows increased absorption of dietary iron and release of iron from stores.8,9 The supply of additional iron from the diet and stores then permits increased hemoglobin synthesis. A single injection of an ESA in humans significantly decreases serum hepcidin within 24 hours,7 but erythropoietin does not appear to be a direct regulator of hepcidin.9 The mechanisms by which erythropoiesis affects hepcidin production are not well understood, but both direct and indirect effects of anemia and erythropoiesis could contribute. Candidate mediators include soluble factors released by erythroid precursors, and decreased circulating or stored iron.10 Hypoxia may alter hepcidin production directly through hypoxia inducible factor (HIF)11 or indirectly via increased erythropoietin production and erythropoiesis.
Hepcidin production is strongly increased by inflammation and infection. The increase appears to be mediated by interleukin-6/signal transducer and activator of transcription–3 (STAT3), as well as other cytokine pathways.12 Because of this, hepcidin levels are elevated in a range of inflammatory disease including rheumatologic diseases, inflammatory bowel disease, a variety of infections, critical illness, and malignancies.8 Increased hepcidin concentrations cause the retention of iron in macrophages and enterocytes, leading to hypoferremia and iron-restricted erythropoiesis.13 Even without any inflammation, overexpression of hepcidin in mice has been shown recently to be sufficient to cause anemia and resistance to high-dose treatment with an ESA.14
Laboratory detection and evaluation
The laboratory diagnosis of absolute iron deficiency has been based on low serum iron, low percent transferrin saturation, and low ferritin.15 However, it has long been known that inflammation can mimic some aspects of iron deficiency by impairing the utilization of existing iron stores for red cell production, and inducing an iron-sequestration syndrome and hypoferremia.16 As described previously, the molecular mechanisms that underlie the redistribution of iron during inflammation center on the cytokine-stimulated overproduction of hepcidin. Iron becomes limiting for erythropoiesis but generally, the resulting anemia is not severe. This could be due to counterregulation of hepcidin by hypoferremia/anemia, but another mechanism related to ferroportin expression in erythrocyte precursors17,18 may contribute. If ferroportin on erythrocyte precursors exports iron, this would require that the cells possess excess capacity for diferric transferrin uptake, over and above the requirements for hemoglobin synthesis. During inflammation, increased hepcidin would degrade ferroportin, the “wasteful” iron export would stop, and the high capacity for differic transferrin uptake would preserve adequate hemoglobin synthesis despite systemic iron restriction.
Serum ferritin has the potential to differentiate true iron deficiency from inflammatory iron sequestration. However, both inflammation and intracellular iron accumulation stimulate the production of the iron storage protein ferritin whose soluble form is detectable in plasma and serum. Therefore, interpretation of serum ferritin in patients with inflammation due to comorbidities is more challenging. The generally accepted cut-off level for serum ferritin to indicate absolute iron deficiency previously has been ≤ 12 ng/mL.19 However, more recent studies correlating the presence or absence of stainable iron with serum ferritin in normal individuals and in patients, and also patients with anemia responsive to iron therapy, indicate that this threshold level of ferritin had only a sensitivity of 25% for detecting iron deficiency.20 The sensitivity could be improved to 92%, with a positive predictive value of 83%, by using a diagnostic cutoff value of ≤ 30 ng/mL.
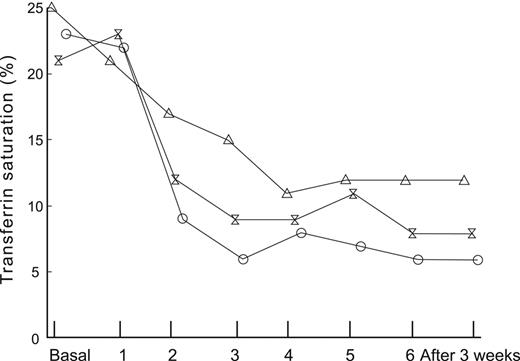
The differentiation among absolute iron deficiency, functional iron deficiency, and iron-sequestration syndromes is important for patient management. Functional iron deficiency is manifest by a fall in iron saturation either during erythropoietin–stimulated erythropoiesis, or with initiation of ESA therapy, as illustrated in Figure 2.21 Absolute iron deficiency may be the presenting sign of occult blood loss from gastrointestinal lesions including malignancy,23,24 whereas an iron sequestration phenotype is indicative of an underlying inflammatory disorder. Commonly used laboratory tests such as serum iron, total iron-binding capacity, mean corpuscular volume, transferrin saturation, and ferritin provide limited diagnostic value.13,25
The impact of endogenous erythropoietin-mediated erythropoiesis or ESA mediated erythropoiesis, on iron saturation and ferritin. Patients undergoing autologous blood donation before elective orthopaedic surgery are shown at baseline and after treatment with placebo or 1 of 2 doses of recombinant human erythropoietin (rHuEPO) at each visit during the donation period. All patients received supplemental oral iron. Mean transferrin saturation in 24 patients receiving placebo, 300 U/kg rHuEPO, or 600 U/kg rHuEPO (θ).21
The impact of endogenous erythropoietin-mediated erythropoiesis or ESA mediated erythropoiesis, on iron saturation and ferritin. Patients undergoing autologous blood donation before elective orthopaedic surgery are shown at baseline and after treatment with placebo or 1 of 2 doses of recombinant human erythropoietin (rHuEPO) at each visit during the donation period. All patients received supplemental oral iron. Mean transferrin saturation in 24 patients receiving placebo, 300 U/kg rHuEPO, or 600 U/kg rHuEPO (θ).21
Increased soluble transferrin receptor (sTfR) has been reported to be an indicator of iron deficiency,26 because sTfR is released by erythropoietic precursors in proportion to their expansion and is not increased by inflammation. However, this assay was found to have a specificity of 84% and a positive predictive value of only 58% in a population of patients likely to be typical of the most difficult diagnostic environments for assessing iron status.20 Interpretation of increased sTfR therefore may be challenging, even in the absence of known causes of increased erythropoiesis other than iron deficiency. Similarly, attempts to combine ferritin and sTfR results (sTfR/log ferritin)27 still fall short when analyzed for diagnostic sensitivity and specificity, and must be corrected for acute phase reactant changes in the setting of inflammation.25
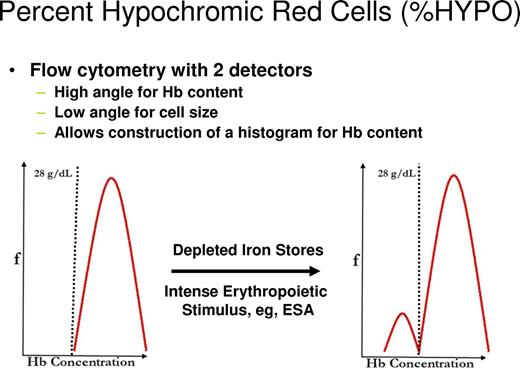
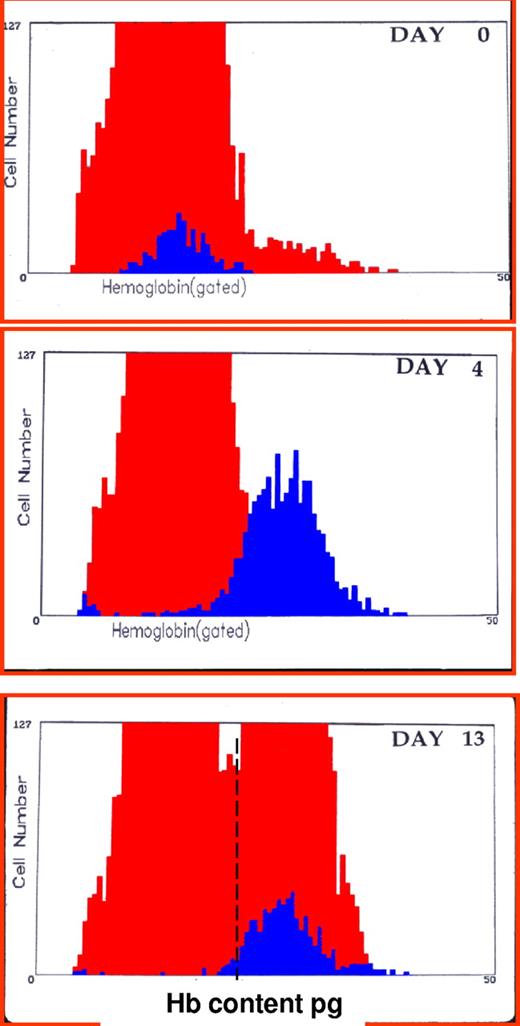
Clinically, it would be helpful to detect the earliest changes in red cell indices that reflect iron-restricted erythropoiesis. One approach would be to identify newly formed iron-deficient cells when they are released from the bone marrow as reticulocytes. Flow cytometric analysis of reticulocytes allows determination of reticulocyte hemoglobin (CHr) content2 or percentage of hypochromic reticulocytes (%HYPO).28 As illustrated in Figure 3, this approach can also identify functional iron deficiency in iron-replete volunteers receiving ESA therapy.29 The %HYPO measure is regarded as a time-averaged marker of iron-restricted erythropoiesis (20-120 days), whereas the CHr measure is a real-time parameter (48 hours).30 As illustrated in Figure 4, hemoglobin content of reticulocytes in iron deficient subjects increases significantly after intravenous iron therapy and reaches normal values by day 4, suggesting that reticulocyte indices allow for real-time evaluation of iron-deficient erythropoiesis and for monitoring response to iron replacement therapy. Many existing laboratory analyzers are capable of measuring CHr but may require modification with software patches.
The effect of depleted iron stores or ESA therapy on flow cytometry detection of %HYPO.
The effect of depleted iron stores or ESA therapy on flow cytometry detection of %HYPO.
CHr: early indicator of iron deficiency and response to therapy. Histogram of red blood cells (gray area) and reticulocytes (black area) hemoglobin content (picograms per cell) before and 4 and 13 days after intravenous administration of iron dextran in a patient unresponsive to oral iron therapy. Values at day 0 were hemoglobin 7.8 g/dL, mean corpuscular hemoglobin 16 pg, reticulocytes 1.8%, CHr 16 pg. Values at day 4 were hemoglobin 8.7 g/dL, mean cell hemoglobin 17.3 pg, reticulocytes 6.4%, CHr 26 pg. Values at day 13 were hemoglobin 11.2 g/dL, mean cell hemoglobin 20.8 pg, reticulocytes 3.4%, CHr 27.1 pg.2
CHr: early indicator of iron deficiency and response to therapy. Histogram of red blood cells (gray area) and reticulocytes (black area) hemoglobin content (picograms per cell) before and 4 and 13 days after intravenous administration of iron dextran in a patient unresponsive to oral iron therapy. Values at day 0 were hemoglobin 7.8 g/dL, mean corpuscular hemoglobin 16 pg, reticulocytes 1.8%, CHr 16 pg. Values at day 4 were hemoglobin 8.7 g/dL, mean cell hemoglobin 17.3 pg, reticulocytes 6.4%, CHr 26 pg. Values at day 13 were hemoglobin 11.2 g/dL, mean cell hemoglobin 20.8 pg, reticulocytes 3.4%, CHr 27.1 pg.2
After initial observations that hepcidin played a major, causative role in patients with anemia due to hepatic adenomas, hepcidin was implicated in the pathogenesis of the anemia of chronic disease in general.31 The development of sensitive, accurate and reproducible immunoassays3 and mass-spectrometric assays32 for human hepcidin has allowed detailed definition of physiologic and pathologic changes of hepcidin in healthy volunteers and in patients. The assays will be useful in improving our understanding of the pathogenic role of hepcidin in various iron disorders, and in the development of appropriate therapeutic interventions. In contrast to ferritin, changes in hepcidin concentrations are the cause of, rather than the result of, iron disorders.
Expected changes in hepcidin levels and iron parameters in various clinical conditions, in iron therapy strategies, and in the potential use of hepcidin-targeted therapies in patients with various forms of anemia, are summarized in Table 2. For example, hepcidin may distinguish patients with functional iron deficiency from those with iron-sequestration syndromes.33 In the former, hepcidin would be expected to be low as a result of the decreased availability of iron for erythropoiesis. Conversely in the latter, hepcidin, as the pathogenic mediator of inflammation, would be expected to be high. Because hepcidin levels are affected by iron stores,3 this assay may also identify patients most likely to respond to iron therapy (if low) or identify patients at risk for iron loading (if high). Whether hepcidin levels can guide iron therapy requires future study.33
It is noteworthy that hepcidin levels are reliably elevated in patients with anemia of inflammation (AI) compared with normal values, and are low or undetectable in patients with iron deficiency anemia (IDA) alone. However, in patients who have mixed AI and iron deficiency (IDA), hepcidin levels may not be reliably distinguished from patients with IDA alone.34 As indicated in Table 2, more complex algorithms will need to be tested to provide optimal guidance for the evaluation and management of patients with mixed presentation.
Management
With the growing recognition that anemia is associated with adverse clinical outcomes in a variety of clinical settings, and that below-normal hemoglobin levels should no longer be regarded as simply abnormal laboratory values,35 how should iron-restricted erythropoiesis be managed? The management of such patients depends on the assessment of whether the patient has absolute iron deficiency, an iron sequestration syndrome, and/or functional iron deficiency. The diagnostic and therapeutic implications for different categories of iron-restricted erythropoiesis are detailed in Table 2.
Oral iron therapy
Even in the best circumstances, oral iron supplementation is poorly tolerated and patients may be noncompliant,36 not only from side effects of oral iron but because of their diagnosis or the therapy they are undergoing for their disease. Nevertheless, in stable patients with mild to moderate anemia in which there is some time for management strategies, and particularly in those patients in whom diagnostic laboratory testing has not definitively ruled out iron-restricted erythropoiesis, a therapeutic trial of oral iron therapy can be recommended.1 In general, absorption of oral iron inversely correlates with hepcidin levels.37,38 Thus, hepcidin measurements may help to determine a priori which patients are good candidates for oral iron therapy. Exceptions may be patients who malabsorb iron because of damage to the intestinal lining such as in celiac disease, and in patients undergoing treatment with proton-pump inhibitors.
In a study of patients with anemia,20 5 of 54 who had bone marrow examinations had absent iron stores indicating absolute iron deficiency. There were an additional 8 patients who were categorized as iron deficient because of their response to oral iron therapy; half of these had serum ferritin ≥ 12 ng/mL and one of the patients had a serum ferritin > 100 ng/mL. This analysis suggests that in the absence of a diagnostic bone marrow examination, no current laboratory test can confidently rule out iron deficiency. Thus, identifying patients who have absolute iron deficiency is essential for successful patient management because it may be a sign of serious underlying illness,39,40 including malignancy.23,24
Failure to respond to a trial of oral iron therapy does not rule out iron-restricted erythropoiesis or even true iron deficiency in the setting of inflammation, as inflammatory hepcidin elevation would cause impaired iron absorption.6 Furthermore, ongoing blood (and iron) losses may exceed even maximal gastrointestinal absorption of iron.41 Clinical situations are often complex, and blood loss, iron-restricted erythropoiesis and high hepcidin levels can coexist in patients in whom absorption of dietary or oral iron supplements is impaired.18 In these instances, and in circumstances of ongoing blood loss,41 intravenous iron therapy may be needed either as a diagnostic trial or as definitive therapy.4
Therapy with ESAs in management of the anemia of chronic renal failure has led to substantial clinical experience in supplemental intravenous iron therapy in this setting.42,43 Hyporesponsiveness to ESA therapy is a common phenomenon44,45 due to a variety of comorbid conditions, but particularly related to functional iron deficiency.21,22 Patients with anemia undergoing dialysis may show suboptimal or no response to oral iron therapy for several reasons. During ESA therapy, although absorption of iron can increase up to 5-fold46 presumably due to hepcidin suppression by increased erythropoiesis, ongoing external iron losses due to hemodialysis and blood testing can exceed the intake.47,48 Furthermore, some patients have poor compliance with iron therapy or significantly reduced gastrointestinal iron absorption. Absorption of oral iron can be enhanced with ascorbate by at least 30%, because it prevents formation of insoluble and unabsorbable compound and reduces ferric iron to ferrous iron.48 Iron absorption and release from stores may be impaired due to high hepcidin levels from diminished clearance by the kidneys, not completely corrected by routine hemodialysis, as well as from inflammation. Indeed, overexpression of hepcidin in mice blocked hematopoietic response even to large doses of ESA.13
Intravenous iron therapy
Intravenous iron administration is recommended in renal dialysis patients undergoing ESA therapy.49 Patients treated with intravenous iron (100 mg twice weekly) achieved a 46% reduction in ESA dosage required to maintain hematocrit (Hct) levels between 30% and 34%, compared with patients supplemented with oral iron.50 To further address the management of iron-restricted erythropoiesis in chronic kidney disease patients undergoing dialysis, a randomized controlled trial evaluated the efficacy of intravenous iron supplementation in patients with ferritin between 500 and 1200 ng/mL.51 The administration of intravenous iron (and increasing the dose of ESA by 25%) resulted in a greater correction of anemia compared with increasing the dose of ESA alone. After the end of the trial, there was greater success in reducing the dose of ESA in the patients receiving intravenous iron, compared with the non–iron–treated arm.
Intravenous iron therapy also improves responses to ESA therapy in patients with inflammatory bowel disease52 compared with responses in a similar patient group who receive oral iron supplementation.53 Decreased CHr is an indicator of the inadequacy of iron supply in the face of increased iron demand stimulated by ESA therapy.25 The requirement for a kinetic balance between iron delivery and level of erythropoietin stimulation may explain the need for intravenous iron supplemental therapy in ESA-treated patients, even those with replete iron stores.49 The clinical response to the combination of intravenous iron and ESA therapy may be attributed to the ability of the parenteral route to circumvent the inflammation-induced block to intestinal iron absorption and to deliver sufficient iron to the reticuloendothelial system, and the ability of ESA therapy to mobilize the iron from the reticuloendothelial system via transferrin into red blood cell precursors.9,54,55 It is not clear how intravenous iron can enhance the effect of ESAs even in the face of apparently increased iron stores. It is possible that the additional iron load in the reticuloendothelial system increases iron efflux from macrophages, perhaps by increasing the translation of ferroportin mRNA.
Currently approved intravenous iron preparations are listed in Table 3.56 The risk-benefit profile of intravenous iron continues to undergo evaluation in renal dialysis patients,49,57 as well as in patients with anemia of chronic diseases.13 Intravenous iron can allow up to a 5-fold erythropoietic response to significant blood loss anemia in normal individuals.58,59 A greater rate of red cell production is probably not possible unless red marrow expands into yellow marrow space, as is seen in patients with hereditary anemias.60,61 One potential limitation to intravenous iron therapy may be that much of the administered iron ends up in the reticuloendothelial system as storage iron, from where it is not readily available for erythropoiesis,61 particularly if hepcidin concentrations are elevated. However, for patients with iron deficiency, 50% of intravenous iron is incorporated into hemoglobin within 3 to 4 weeks.62 For patients with anemia of chronic disease or renal failure, intravenous iron is mobilized less rapidly from the reticuloendothelial system.63 Nevertheless, when iron dextran was given intravenously to patients with the anemia of rheumatoid arthritis, cellular hemoglobin concentrations increased significantly.64
In patients with malignancy and chemotherapy-induced anemia, 5 trials have studied intravenous iron in the setting of therapy with ESAs. In one study65 of patients undergoing chemotherapy, 155 patients were treated with ESA and randomized to receive either no iron; oral iron as 325 mg if ferrous sulfate twice daily; 100-mg boluses of intravenous iron dextran weekly until the total calculated iron deficit was administered; or a single treatment of intravenous iron dextran to the same calculated dose. There were significant improvements in hemoglobin levels and hematopoietic responses in both patient groups treated with intravenous iron arms, compared with those receiving oral iron or no iron therapy.
Another study66 assigned 189 patients to receive ESA weekly plus no iron; oral iron as 325 mg of ferrous sulfate thrice daily; or intravenous ferrous gluconate as 125-mg weekly boluses. The cohort treated with intravenous iron had improved hemoglobin and hematopoietic responses compared with the other cohorts. A third study67 randomly assigned 67 patients with lymphoproliferative malignancies not receiving chemotherapy with intravenous or no iron. Again, intravenous iron resulted in improved hemoglobin and hematopoietic responses. A fourth study of 398 patients with chemotherapy-induced anemia who were treated with ESA therapy, found significant improvement in hemoglobin levels and hematopoietic responses in the patient cohort treated with intravenous iron,68 further confirmed by a fifth study.69
Iron-restricted erythropoiesis has been shown to be a consideration at time of cancer diagnosis even before ESA therapy: 17% of carefully screened patients were found to have serum ferritins < 100 ng/mL and 59% had transferrin saturations (TSAT) less than 20% at diagnosis70 In addition, renewed attention has been placed on the dose-response relationship between ESA dosage and red cell production responses in ESA-treated patients.15 Recent controversies regarding the safety of ESA therapy in patients with malignancies and chemotherapy-induced anemia71 have led to the addition of a black box warning as well as more restrictive indications.72 Once ESA therapy is administered, even subjects with normal baseline levels develop TSAT and ferritin decreases to levels indicating iron-restricted erythropoiesis.21,22 Accordingly, guidelines by the National Comprehensive Cancer Network have recommended that iron studies be obtained at baseline to identify patients who are candidates for supplemental iron therapy, and that if subsequent hemoglobin levels after 4 weeks of ESA therapy indicate no response (< 1 g/dL increase in hemoglobin), then intravenous iron supplemental therapy should be considered, along with an increase in ESA dose.73
Even under the best of circumstances, oral iron is not well-tolerated and patients are noncompliant.36 With acute or chronic diseases of inflammation, many patients already have side effects because of systemic inflammation, psychologic aspects of the illness, or because of the therapy that they are undergoing for the disease. In addition, hepcidin response in inflammatory conditions inhibits gastrointestinal absorption of oral iron. While recent evidence in rodents suggests that in the presence of both iron deficiency and inflammation, hepcidin levels are more responsive to the erythropoietic demands for iron than to inflammation, the clinical trials in cancer patients reviewed above65-69 have demonstrated that intravenous iron therapy is more efficacious than oral iron in treating iron-restricted erythropoiesis in oncology patients. In addition, in nephrology patients intravenous iron has been shown to be effective in treating iron-restricted erythropoiesis due to functional iron deficiency in those who have suboptimal responses to ESA therapy. Despite these beneficial effects in treatment of iron deficiency, intravenous iron administration may generate oxidative stress and other inflammatory changes.49 Recently, a novel mouse model showed that red cell transfusion of aged blood resulted in oxidative stress and inflammatory cytokine secretion in response to macrophage-ingested iron from membrane-encapsulated hemoglobin, but not from stroma-free lysate derived from stored red cells.74 Long-term effects of the intravenous iron preparations will require careful study in relevant clinical settings.75,76
Conclusion
In summary, how should the bedside clinician proceed with management of iron-restricted erythropoiesis? On one hand, the hypoferremia and anemia of inflammation can be viewed as a mechanism of defense against providing iron to unwanted pathogens,77 and therefore adaptive. On the other hand, others are not convinced that iron deficiency or iron-restrictive anemia should be regarded as a desirable condition that benefits patients with infection or inflammation. Although more evidence is clearly needed, we recommend that in patients who show symptoms attributable to moderate or severe anemia of inflammation, and do not suffer from overwhelming or difficult to control infection, iron-restricted erythropoiesis can and should be treated, an argument that is not new.78 In iron-restricted anemias other than absolute iron deficiency, the optimal treatment is still evolving and may be improved by the ongoing development of hepcidin-targeted therapies.
Important clinical subjects for further study in this area should include improving the diagnosis of various forms of iron-restricted anemia in complex clinical settings, testing optimal combinations of ESA and intravenous iron treatments, analysis of the relative risk-benefits of ESA and intravenous iron therapy, and the appropriate use of adjuncts such as anti-inflammatory therapy or frequent dialysis. Basic scientific insights that could facilitate the progress in this area include understanding the molecular nature of the erythropoietic iron regulator and suppressor of hepcidin, details of the mechanisms by which circulating and stored iron regulates hepcidin, the contribution of hepcidin-independent mechanisms to anemia of inflammation, and the role of genetics in sporadic iron deficiency.
Acknowledgments
This work was supported by a grant from Amgen and the Roche Foundation for Anemia Research.
Authorship
Contribution: L.T.G., E.N., and T.G. participated in writing, reviewing, and editing the manuscript, including the final draft.
Conflict-of-interest disclosure: L.T.G. is a consultant for Amgen, Luitpold, and AMAG Pharmaceuticals; E.N. is the cofounder and officer of Intrinsic LifeSciences LLC; and T.G. is a consultant for Xenon Pharmaceuticals and part-owner and Chief Medical Officer for Intrinsic Lifesciences LLC.
Correspondence: Lawrence Tim Goodnough, Pathology and Medicine, Stanford University, 300 Pasteur Dr, Rm H-1402, 5626, Stanford, CA 94305; e-mail: ltgoodnough@stanfordmed.org.