Abstract
The use of allogeneic hematopoietic cell transplantation (HCT) has expanded progressively, facilitated by the increasing availability of unrelated donors and cord blood, and the inclusion of older patients as transplantation candidates. Indications remain diagnosis-dependent. As novel nontransplantation modalities have been developed concurrently, many patients come to HCT only when no longer responding to such therapy. However, patients with refractory or advanced disease frequently relapse after HCT, even with high-dose conditioning, and more so with reduced-intensity regimens as used for patients of older age or with comorbid conditions. Thus, patients with high-risk malignancies who have substantial comorbidities or are of advanced age are at high risk of both relapse and nonrelapse mortality and should probably not be transplanted. Being in remission or at least having shown responsiveness to pre-HCT therapy is generally associated with increased transplantation success. In addition, to handle the stress associated with HCT, patients need a good social support system and a secure financial net. They must be well informed, not only about the transplantation process, but also about expected or potential post-HCT events, including graft-versus-host disease and delayed effects that may become manifest only years after HCT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers potentially curative therapy for various congenital or acquired malignant and nonmalignant lymphohematopoietic diseases. However, expected toxicity associated with the transplantation procedure has generally prevented older patients and patients with comorbid conditions from being considered for HCT. Second, graft-versus-host disease (GVHD), the most frequent complication after allogeneic HCT, can cause considerable morbidity and even mortality both early and late after HCT. Third, relapse in patients with high-risk, advanced, or refractory disease has limited the overall success of HCT.
The recent development of transplantation conditioning regimens of lower intensity has allowed HCT to be carried out with less toxicity than observed in the past and has permitted transplantation of patients who hitherto had not been considered candidates. There is some evidence that the use of lower intensity regimens may also alleviate the severity of GVHD.1 One potential drawback is the higher risk of relapse, which, in patients with high-risk disease, may raise concerns about futility.
This Perspectives article will focus on lymphohematopoietic malignancies in adults and will review some current indications for and results of HCT and assess the impact of disease and patient characteristics on transplantation outcome. We will try to put available data into perspective and raise questions that cannot be answered satisfactorily at this point.
Recent developments
Historically, patients who underwent HCT for malignant lymphohematopoietic diseases had exhausted other treatment modalities. The question was not really whether a patient was “fit” for HCT, but rather whether HCT was fit for the patient. Donors were almost exclusively human leukocyte antigen (HLA)–identical siblings, and bone marrow was the only source of hematopoietic stem cells.
Conditions have changed dramatically. The use of HCT has been expanded progressively to better risk and increasing numbers of patients. In 2009, more than 6500 allogeneic HCTs were carried out in North America, and more than 26 000 worldwide. At least in part, this expansion was related to the development of unrelated donor registries and the refinement of HLA typing, which now allows to select unrelated donors matched at the DNA level,2 the use of cord blood cells,3,4 and, most recently, the inclusion of haploidentical donors.5,6 In parallel, there was a rapid increase in the use of granulocyte colony-stimulating factor–mobilized cells harvested from peripheral blood (PBPCs). This strategy avoids the need for donor anesthesia, and PBPCs have been shown to result in more rapid engraftment, albeit at the expense of a higher incidence of chronic GVHD.7,8
Furthermore, a cursory review of the literature shows that the median age of transplanted patients has increased continuously over the past few decades. While the upper age limit for HCT for many years was 50 or 55 years, recent reports include patients in their 70s. The Center for International Blood and Marrow Transplantation Research (CIBMTR) database shows a median patient age of 25 years in the 1980s, 39 in the 1990s, and 46 over the past decade. From 2002 to 2009, 44% of patients were older than 50, and 20% older than 60 years (ie, every fifth patient was in the seventh or eighth decade of life).
Transplantation of progressively older patients was made possible largely by the development of low-intensity conditioning regimens tolerable by older patients. Although dose intensification of conditioning regimens, as practiced in the past, had been shown to be effective in reducing relapse incidence, it resulted in unacceptable nonrelapse mortality (NRM).9 Thus, based on observations by several investigators in preclinical models and in clinical pilot studies,10-12 new efforts placed the emphasis on the exploitation of immune effects mediated by donor cells (the graft-versus-leukemia [GVL] effect) while reducing the intensity and, as a result, the toxicity of conditioning regimens. Reduced/low-intensity regimens, such as fludarabine combined with low-dose total body irradiation (TBI; 200 cGy), busulfan (eg, 8 mg/kg), or melphalan (eg, 2 × 70 mg/m2), have consistently allowed for sustained donor cell engraftment. Concurrently, more intensive conventional regimens have been modified with the goal of reducing toxicity while maintaining or improving efficacy.13,14 Those strategies have included the replacement of high-dose TBI by chemotherapeutic agents, substituting drugs with little nonhematopoietic toxicity for agents with broader organ toxicity or incorporating agents such as thymoglobulin into the regimen.15-18 Another approach involves the conjugation of radioactive isotopes, such as iodine-131 or yttrium-90, to monoclonal antibodies (anti-CD20, anti-CD45) specific for antigens restricted to hematopoietic or lymphoid cell populations of interest.
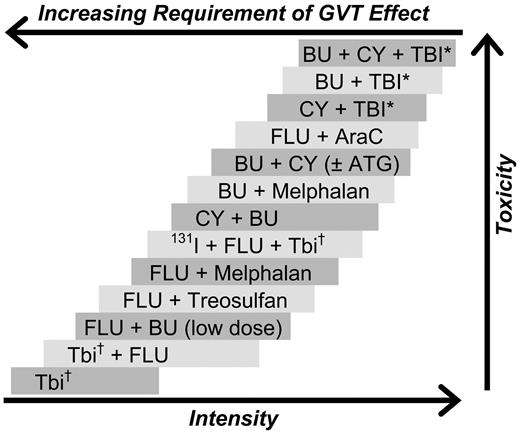
As a result, we have at our disposal a broad spectrum of regimens suitable for various disease categories and patient populations (illustrated in Figure 1). Attempts at classifying regimens as belonging to distinct intensity categories, although convenient, appear somewhat artificial.19
Selected conditioning regimens of different dose intensities. BU indicates busulfan; CY, cyclophosphamide; TBI, total body irradiation; Flu, fludarabine (various dosing schedules); AraC, cytosine arabinoside; ATG, antithymocyte globulin (or thymoglobulin); and 131I, anti-CD45 antibody conjugated to 131I. *“High-dose” TBI (800-1320 cGy). †“Low-dose” TBI (200-400 cGy).
Selected conditioning regimens of different dose intensities. BU indicates busulfan; CY, cyclophosphamide; TBI, total body irradiation; Flu, fludarabine (various dosing schedules); AraC, cytosine arabinoside; ATG, antithymocyte globulin (or thymoglobulin); and 131I, anti-CD45 antibody conjugated to 131I. *“High-dose” TBI (800-1320 cGy). †“Low-dose” TBI (200-400 cGy).
The availability of reduced-intensity regimens has also allowed for second transplantations to be carried out after failure of a previous (autologous or allogeneic) transplant after a high-dose regimen, a situation where repeat conditioning with high-dose therapy is expected to cause prohibitive toxicity.20-22
Factors to consider when counseling patients on transplantation
Some generally agreed-on eligibility criteria for HCT are summarized in Table 1. Pretransplantation, transplantation-associated, and posttransplantation risk factors cannot be separated completely, as pretransplantation conditions will affect selection of the transplantation protocol, and both may modify posttransplantation events, such as GVHD incidence and severity or infections.
Many pre-HCT factors are “givens” that we cannot change, such as type of disease, patient age, and genetic determinants. The same applies to most comorbid conditions. Assuming a suitable donor is available, we have some control over the timing of HCT and thereby the disease stage when HCT is carried out. However, those decisions may be challenged in view of ongoing developments of novel nontransplantation therapy for patients with lymphohematopoietic malignancies. Indeed, patients referred for HCT in recent years may have been exposed to chemotherapeutic or biologic agents whose impact on HCT is unknown; conceivably, patients who have received these regimens are more refractory to the effects of HCT than patients exposed to different agents in the past.
The choice of transplantation strategy is largely determined by pre-HCT risk factors, but also by the pursuit of research questions aimed at further improving results. Currently, older patients and patients with comorbidities will generally be offered HCT using reduced/low-intensity conditioning. However, as available data show a higher relapse rate with reduced/low-intensity conditioning in several disease categories,23 the disease status becomes a central issue, and often the decision is to give “debulking” therapy before HCT to increase the probability of post-HCT success.22,24 There are no controlled prospective data to assist in decision-making. However, the success of high-dose conditioning/autologous HCT followed by low-dose conditioning/allogeneic HCT in patients with multiple myeloma or high-grade lymphomas illustrates the potential of debulking therapy.25
GVHD, primarily determined by histoincompatibility between donor and patient, has remained the major post-HCT risk factor for long-term outcome.26,27 Hence, selection of HLA-matched donors remains the preferred approach by most transplantation centers, although trials using HLA-incompatible cord blood or HLA-haploidentical donors show encouraging results.3,6,28 However, the development of GVHD, which occurs in its acute or chronic form in at least half of the patients, even with complete HLA matching, is also affected by the intensity of the conditioning regimen,29,30 possibly by pre-HCT therapy, by donor allosensitization,31,32 and, as suggested in recent reports, by factors such as donor (and patient) treatment with statin drugs.33 So far, virtually all published reports are based on retrospective analyses.
Disease characteristics and transplantation outcome
Disease stage and cytogenetic abnormalities have consistently been identified as the strongest determinants of relapse after HCT. This is true regardless of patient age or comorbid conditions, although some studies in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) showed higher relapse rates in older than in younger patients. Whether differences are related to age-dependent differences in disease biology or differences in pre-HCT treatment is a matter of debate.
Implicit in any decision for or against HCT is, of course, the question of superiority of HCT results over nontransplantation strategies. More specifically, how much better do results with HCT have to be to justify the potential risks (and costs) associated with HCT? Is a statistically significant difference sufficient or do we need to focus on clinical significance, and how do we measure it?
Koreth et al34 presented a meta-analysis involving 6000 adults with AML in first remission enrolled in 24 prospective trials between 1982 and 2006. The upper age limit ranged from 40 to 60 years, and patients could have no significant comorbidities. Conditioning was with high-dose regimens. The objective was to determine to what extent HCT from HLA-identical siblings improved overall and relapse-free survival (RFS) compared with patients without donors who were randomized to receive chemotherapy or autologous HCT. Compliance with allogeneic HCT was generally more than 60%. The hazard ratio (HR) for death or relapse with allogeneic HCT was 0.80, indicating a statistically significant benefit of HCT across all cytogenetic groups (P < .01). However, the benefit was restricted to patients with poor/intermediate-risk cytogenetics (HR = 0.69 and 0.76, respectively); no benefit was apparent for good-risk cytogenetics (HR = 1.06). Autologous HCT was not superior to chemotherapy. The 5-year overall survival with poor- and intermediate-risk cytogenetics was 31% and 52%, respectively, in the allogeneic transplantation group, compared with 20% and 45%, respectively, in patients without an allogeneic donor. Thus, depending on cytogenetics, patients with allogeneic donors had survival probabilities 7% to 11% higher than nontransplanted patients. This analysis included only studies that used high-dose conditioning and sibling donors for patients younger than 60 years. Whether results would be similar in older patients or those transplanted from unrelated donors could not be addressed. Furthermore, in regard to “selection bias,” even donor versus no donor analyses are not without problems.35
Gyurkocza et al recently presented data on 274 patients, 5 to 74 years of age (median, 60 years), with AML in first or second remission or relapsed/refractory disease.36 Among 211 patients with classifiable karyotype, 5% had better-risk (favorable) cytogenetics, 31% worst-risk (Southwest Oncology Group criteria), and 43% intermediate-risk cytogenetics. Conditioning consisted of TBI, 200 cGy with or without fludarabine, 3 × 30 mg/m2. Donors were related (n = 118) or unrelated, and 88% were HLA-matched with the patients. With a median follow-up of 38 months, the projected 5-year survival was 37% and 34% for patients in first and second remission, respectively, and 18% for patients with advanced disease. Incidence of relapse or disease progression was 42%. Worst-risk cytogenetics were correlated with increased relapse probability and reduced RFS. NRM was related primarily to GVHD and infections and was 4%, 16%, and 26% at 100 days, 1 year, and 5 years, respectively. No plateau of survival was observed. Of note, age, AML etiology, and pre-HCT hematopoietic recovery did not significantly affect outcome. There was a trend for comorbidities (by Charlson Comorbidity Index for use in HCT [HCT-CI]) to increase NRM (P = .08).37,38 Although this multi-institutional analysis did not compare HCT with chemotherapy results, a 5-year survival of 18%, even for patients with refractory disease, indicates that there are patients with chemotherapy-refractory AML who are cured by allogeneic HCT, presumably via an immunologic graft-versus-tumor (GVT) effect. This observation is consistent with an earlier study from the M. D. Anderson Cancer Center, which showed that older patients referred for HCT fared better than patients who received only chemotherapy. A central question is how to select those patients. As with high-dose conditioning, the most relevant parameter determining post-HCT survival in older patients conditioned with low-intensity regimens was the patient's karyotype (ie, patients with poor-risk karyotype did poorly). One difference, however, with lower intensity regimens is the greater dependence of leukemia eradication on the donor cell-mediated GVT effect; relapse probability was, indeed, lower in patients with chronic GVHD. These data and additional studies39 indicate that no conditioning regimen has yet been able to negate the impact of disease risk category on transplantation outcome. The observation that similar prognostic factors (cytogenetics, Flt3 ITDs) affect relapse rates after both HCT and chemotherapy40-42 suggests that these modalities differ quantitatively more than qualitatively.
Another disease with poor prognosis in adult patients is acute lymphoblastic leukemia (ALL).43,44 Goldstone et al recently presented the results of an international ALL trial (MRC UKALL XII/ECOG E2993), which from 1993 through 2006 enrolled almost 2000 patients, 15 to 59 years of age.45 Patients with HLA-identical siblings were assigned to allogeneic HCT, whereas those without a donor (and patients older than 50 or, later in the study, 55 years) were randomized to autologous HCT or continued chemotherapy. Patients older than 35 years, patients who were Philadelphia chromosome–positive (Ph+) or presented ≥ 30 × 109 cells/L of T lineage or ≥ 100 × 109 cells/L of B lineage were considered “high risk.” The 5-year survival of 1913 eligible patients was 39%; it was 43% for Ph− patients, the cohort included in the final analysis. Five-year survival was 53% for patients with, and 45% for patients without a donor (P = .01). The relapse rate was significantly lower in patients with donors (P ≤ .001). However, in the high-risk group, NRM was 36% among patients with allogeneic donors, versus 14% in the remaining patients; 5-year survival did not differ significantly (41% vs 35%; P = .2). Among standard risk patients, NRM was 20% (donor) and 7% (no donor), respectively, and the corresponding survival was 62% and 52%, respectively (P = .02). These data suggest that standard-risk patients are more probable to benefit from HCT. Although the causes of death among transplanted and nontransplanted patients differed, no significant survival advantage with HCT was observed among higher risk patients, and probably those patients should not be transplanted, unless on a clinical trial investigating new strategies aimed at reducing the risk of NRM.
Among Ph+ patients, 28% proceeded to HCT in first remission. Overall survival at 5 years was 44% after sibling donor HCT and 36% for HCT from unrelated donors (which was allowed for this cohort). An intent-to-treat analysis showed 5-year survivals of 34% and 25% for patients with and without donors, respectively (P = .001).46 Thus, in contrast to adult patients with Ph− ALL, among whom only those with standard risk did benefit from allogeneic HCT, among Ph+ patients, a modest but statistically significant survival advantage was achieved with allogeneic HCT.
In chronic lymphocytic leukemia refractory to or relapsing after treatment with fludarabine and cyclophosphamide, HCT from related or unrelated donors after low-intensity conditioning regimens resulted in progression-free survival of approximately 60%,47 although bulky lymph node disease had a negative impact on outcome. Khouri et al48 reported results in 47 patients with relapsed follicular lymphoma conditioned with fludarabine, cyclophosphamide, and rituximab, followed by the infusion of PBPC or marrow cells from related (n = 45) or unrelated donors (n = 2). With a median follow-up of 5 years, RFS was 83%. These results indicate that patients with “indolent” lymphoid malignancies may have an excellent chance of cure by HCT, even if not in remission at HCT. Another report on patients with heavily pretreated or refractory49 non-Hodgkin lymphoma, conditioned with 2 Gy TBI with or without fludarabine and transplanted with PBPCs showed a 3-year progression-free survival of 43% for patients with indolent disease but 21% with transformed disease. Overall, these data suggest that HCT can be curative for patients with non-Hodgkin lymphoma, even if not in remission at the time of HCT, but HCT should be carried out before disease transformation occurs.
The diagnosis for which a patient is to be transplanted or, rather, the therapy received before HCT may also affect the patient's fitness. Those considerations overlap, of course, with disease stage. A patient with AML in first remission may have received one induction course and 2 or 3 consolidation courses, whereas a patient in persistent relapse very likely received twice that amount of therapy before HCT. These differences may be even more striking among patients with lymphoma in first remission or after multiple relapses and various types of combination chemotherapy, involved field irradiation, or even previous autologous HCT.
The dilemma as to the optimum timing of allogeneic HCT is that patients with a particular diagnosis may be cured of their disease with chemotherapy alone and would not derive benefit from HCT, whereas those who are not cured with nontransplantation therapy are also less likely to do well with HCT.
Some general guidelines regarding HCT have been proposed for various disease categories (Table 2). However, there is considerable variability and controversy. In addition, with the identification of prognostically relevant molecular markers, new risk categories are being defined. The presence of such markers (eg, Flt3 or c-kit mutations) might lead to the conclusion that patients with “standard risk” AML by conventional criteria should be transplanted in first remission because expression of the respective markers is associated with poor prognosis.50-54 Although transplantation appears to improve outcomes in patients with markers, such as Flt3 mutations, those markers are also associated with inferior outcome after HCT.55
Age and transplantation
As discussed, the average age of patients undergoing HCT has risen over the past 3 decades. Whereas in younger patients decisions regarding HCT are governed by disease risk and donor availability, in older patients the presence of comorbid conditions and the suitability of conditioning regimens play a central role.
The European Bone Marrow Transplantation group recently reported results in 1333 patients, 50 to 74 years of age, with MDS or secondary AML who were transplanted from HLA-identical siblings (n = 811) or unrelated donors (n = 522); 883 patients were 50 to 60 years of age, and 449 were older than 60 years. Estimated 4-year survival for all patients was 31%. Advanced disease (P < .01) and reduced-intensity conditioning (P < .01) correlated with relapse; disease stage (P = .01), use of unrelated donors (P = .03), and reduced-intensity conditioning regimen (P = .03) were associated with NRM. Age showed no significant association with outcome.56
The CIBMTR presented data on 1080 patients more than 40 years of age with MDS or AML in first remission, transplanted in 1995 to 2005 after conditioning with reduced-intensity conditioning regimens.57 Two-year survival for patients with AML ages 40-54, 55-59, 60-64, or older than 64 years was 44%, 50%, 34%, and 36%, respectively (P = .05). Multivariate analyses showed a negative effect of unfavorable cytogenetics on relapse and RFS, and 2-year overall survival was correlated with pre-HCT performance status. Chronologic age did not impact NRM, relapse, or GVHD, leading the authors to conclude that chronologic age alone should not be a contraindication to HCT.
This observation is of interest as AML in older persons is frequently associated with high-risk features, in particular unfavorable karyotype.58 It is probable, however, that the number of patients in the oldest subcohort was too small and the average age still too young (compared with nontransplantation trials) to observe a significant negative impact of those features.
Biologic age, however, must be assessed in the decision process, although agreeing on parameters that define acceptable biologic age is difficult. It is important to consider how well (or poorly) a patient is expected to tolerate not only the transplantation regimen but also the management after HCT, in particular GVHD and its treatment. Beginning at approximately age 40, the glomerular filtration rate declines by approximately 1 mL/year (ie, at age 70), the baseline glomerular filtration rate may be 60 instead of 90, and the kidney response to nephrotoxic agents may be more profound than in younger patients.59,60 Indeed, older age has been reported to be a risk factor for the development of chronic kidney disease after HCT.61 Similarly, a loss of approximately 30 mL/year has been described for the first second forced expiration volume, which might interfere with airway clearance and infection prevention after HCT. Therefore, even in the absence of clinically apparent comorbidities, changes in organ function with age may modify drug metabolism or excretion and contribute to differences in outcome, compared with younger patients.
Another important difference: whereas younger patients, not surprisingly, are looking for many more years of life, older patients may feel very strongly about quality of life (QOL) during whatever time remains. QOL, however, can be significantly impaired by transplantation-related complications, in particular GVHD and its treatment.
Comorbid conditions and transplantation outcome
In addition to risk classification systems for particular diseases, several indices of comorbidity have been developed and evaluated in efforts to predict, regardless of the specific disease, whether a patient is likely to do well after HCT. Chien et al presented a predictive index, referred to as Pretransplantation Assessment of Mortality (PAM), primarily based on lung function.62,63 Sorror et al modified the established and more broadly applicable HCT-CI by incorporating additional parameters with greater relevance in HCT and by assigning scores (1, 2, or 3) with better discriminating power to individual parameters.64 The HCT-CI strictly considers only pretransplantation patient characteristics, in particular cardiovascular, gastrointestinal, hepatic, and renal dysfunction along with antecedent solid cancer.
Numerous reports have shown increased mortality and lower survival with increasing HCT-CI scores. Patients with scores of 3 or higher generally had significantly inferior survival, often half the probability of patients without comorbidities. A score of 3 could result, for example, from a combination of atrial fibrillation with diabetes mellitus and a body mass index of more than 35 or an active infection in a patient with a creatinine of more than 177μM or a prior diagnosis of breast cancer. In an analysis of results in patients with chronic myelomonocytic leukemia, a score of 3 or higher reduced the probability of long-term survival from 54% to less than 20%.65 An analysis of results in 391 patients with AML and 186 patients with MDS transplanted after conditioning with low- (n = 125) or high-dose regimens (n = 452)66 showed 2-year overall survivals of 70% and 57% for patients with HCT-CI scores of 0-2 and low- or high-risk disease, respectively, after conditioning with either low- or high-intensity regimens. Patients with HCT-CI scores of 3 or higher, with low-risk or high-risk disease, had survivals of 41% and 29%, respectively, after low-intensity conditioning, and 45% and 24%, respectively, after high-intensity regimens. The only difference was higher relapse rates with low-intensity regimens (P = .05). Thus, data from this (retrospective) analysis indicate that comorbidity scores rather than regimen intensity determined outcome and that low-intensity conditioning regimens did not improve outcome over that observed with high-intensity regimens. Similar data have been presented by others,67-69 including patients undergoing autologous HCT,70 although not all studies validated the HCT-CI.71 These are important observations, which show that the patient's condition, including comorbidities and performance status, which presumably reflects biologic age, is of equal importance to disease status when assessing a patient's suitability for HCT.72
Assessment of pulmonary fitness for HCT has often been based on the diffusing capacity of carbon monoxide (DLCO), using a value of 60% as the acceptable limit.73 The pulmonary function-based PAM score also includes transplant donor type, disease risk, and planned transplantation regimen, and as such provides a more functional score. One analysis showed that the discriminating capacity of PAM was superior to DLCO and that patients with DLCO less than 60% could be transplanted successfully,73 an important finding, for example, for patients with autoimmune diseases involving the lungs who are considering HCT. One study compared the PAM model with the HCT-CI and found that a modified HCT-CI, allowing for risk group stratification different from that used in the original description, was the best predictor of NRM.74 An additional analysis involving data on 286 patients transplanted at a French center found the comorbidity indices to have little discriminating power but acknowledged that many children did not have pulmonary function tests available.75
Several analyses examined the impact of patient weight on transplantation outcome.76-78 Although not without controversy, transplantation success in patients who are severely underweight or obese appears to be inferior to that achieved in patients closer to ideal body weight.78-80 Inferior outcome in underweight patients was presumably related to aggressive pre-HCT therapy, which led to negative nitrogen balance and wasting. The reasons are less clear in severely overweight patients. However, these patients tend to be less active, are more difficult to care for when severely ill and unable to cooperate, and may be more prone to develop infections. In addition, differences in the volume of distribution of drugs and the impact on pharmacokinetics81 have been considered as possible explanations.
Other parameters may be more difficult to quantify. In addition to pretransplantation depression (which is considered in the HCT-CI), the extent of social support and female gender have been found to affect physical recovery and emotional stability after HCT.82
Race and socioeconomic status
A recent report from the Institute of Medicine addressed the issue of unequal access to medical treatment, dependent on race and ethnicity.83 However, race, as emphasized recently,84 is a complex social, cultural, and political construct, not a biologic concept. In any event, an earlier study, summarizing data from 4 states, showed that blacks were less likely than whites to undergo HCT for leukemia or lymphoma.85 This was confirmed in a second study,86 although another report found no difference.87
However, once the patient is referred for HCT, how would black race lead to a negative impact on transplantation outcome?88-90 In an analysis of 2221 allogeneic transplantation recipients, black race was associated with significantly higher mortality rates than observed in whites (HR = 1.65).89 Transplantation both from related and unrelated donors was associated with more severe acute GVHD and higher NRM. Yet a matched cohort analysis showed no correlation of higher mortality with socioeconomic status, leading the authors to suggest that differences may be the result of currently unidentified genetic polymorphisms. A second analysis used data on 6207 patients of different races, transplanted from unrelated donors and reported to the CIBMTR.88 The analysis focused on socioeconomic status. Patient income was estimated from residential zip codes at the time of HCT. A Cox regression analysis, adjusting for other risk factors, showed that blacks (but not Hispanics or Asians/Pacific Islanders) had inferior overall survival after HCT compared with white patients (relative risk [RR] = 1.47; P < .001). NRM was higher in blacks (RR = 1.56; P < .001) and Hispanics (RR = 1.30; P = .001). Across all racial groups, patients in the lowest quartile of median income had worse overall survival (RR = 1.15; P = .005) and higher risk of NRM (RR = 1.21; P = .002) than patients with higher incomes. Similar to the first report, the authors concluded that inferior transplantation outcome in blacks is not fully explained by socioeconomic status; other factors, such as genetic polymorphisms and health behavior, may contribute. It appears possible, however, that comorbid conditions (eg, hypertension and kidney disease, which are prevalent in blacks), negatively impacted outcome. Studies are needed to address these factors. Nevertheless, regardless of race, low socioeconomic status had a negative impact on the success of HCT. Low socioeconomic status is probably correlated with lack of health insurance, which may be associated with poor health maintenance, and the need to delay HCT. Postponement or delayed referral is probably associated with more advanced disease, although such an explanation was not supported by the analysis by Mielcarek et al.89 Lastly, from a very practical viewpoint, dark skin complexion, as in blacks, may render the interpretation of skin findings difficult; for example, the diagnosis of cutaneous GVHD may not be made in a timely fashion and possibly go untreated.
Financial fitness
Decisions about patient treatment must be guided by medical considerations. However, health care is not an unlimited resource,91 and the patient's own financial reserve may be limited as well. Coverage for health care differs from country to country; the United States has, so far, not established a clear system of resource allocation. Although many patients have health insurance that would cover HCT, socioeconomic and demographic factors are likely to determine insurance coverage and, as a result, influence the decision about HCT.92 Furthermore, even patients with insurance coverage often have a lifetime maximum coverage on insurance contracts, and the cost for allogeneic HCT and subsequent medical care may exceed that maximum. As a result, patients may incur major out-of-pocket expenses, thereby exhausting their savings, or even being forced to sell their home. Expenses are due not only to direct medical costs, but also the need for the patient's partner or other family members, serving as caregivers, to take a leave from work, thus incurring a loss of income and the frequent need to rent an apartment close to the medical center to allow for appropriate treatment of the patient. Therefore, a family may make an enormous investment for a probability of long-term success that may be less than 10% or even 5% in high-risk patients, particularly if they have substantial comorbidities in addition to high-risk disease. HCT may not be advisable in this situation.
Evaluation of results: who should and who should not be transplanted?
The goal of medical treatment is to provide effective therapy safely. Extensive clinical research over the past decades has rendered HCT progressively safer, in part related to efforts directed at optimizing conditioning regimens, and we can expect the field to continue to evolve, eliminating some of the problems that currently exist. At present, relapse, a disease-intrinsic problem, and GVHD, a treatment-related (ie, iatrogenic) complication, remain major problems27,93 ; because of the GVHD-associated GVT effect, these problems are closely interrelated. As the intensity of transplantation conditioning is reduced, we rely increasingly on donor cell-mediated GVT effects to prevent relapse. Reduced conditioning intensity, however, may also be associated with altered cytokine profiles and lessened GVHD, which might weaken the GVT effect. Indeed, for certain disease categories, relapse rates have been higher after reduced-intensity than after high-intensity conditioning.30,36
Although it is impossible to offer guidelines that apply across the board, some general recommendations are summarized in Table 2; all those patients deserve consultation with a transplantation physician. Of course, other patients not meeting those criteria who want to explore the transplantation option should also be evaluated. However, patients with chemotherapy-refractory disease, who are only candidates for reduced-intensity conditioning, are generally (there are always exceptions) not good candidates for allogeneic HCT. This limitation applies to patients with substantial comorbidities and to older patients. Not every 70-year-old is a candidate for allogeneic HCT, although there are persons who are biologically younger than their chronologic age and for whom HCT should be considered if (1) they have no significant comorbidities, (2) stable social and economic support is present, (3) their disease is considered “responsive,” and (4) the patient understands fully the potential complications and long-term sequelae that may ensue.94
Even younger patients, and patients without significant comorbidities, whose disease has been refractory to various chemotherapeutic regimens, are not good candidates for current transplantation strategies except, possibly, as part of clinical trials that address refractory disease with disease-specific approaches. These patients may be able to tolerate high-dose conditioning regimens that are currently available, and NRM may not be excessive, but HCT may not offer a significant advantage over nontransplantation therapy (see, for example, the discussion of patients with ALL with poor-risk cytogenetics).
Is there an absolute cutoff for comorbidities above which HCT should not be offered, even with the use of low-intensity conditioning regimens? The answer depends on what we accept as minimal success rate, in its own right, and compared with nontransplantation therapy. Patients with severely impaired pulmonary function may face a probability of NRM of 80% or higher.63 If such patients also have a disease with high relapse risk, the probability of post-HCT survival in remission approaches zero, thereby rendering allogeneic HCT a futile effort. The recommendation for such patients should be for more conservative management with emphasis on QOL. Physicians are often reluctant to make this recommendation, at least in part, because of concern that they might be denying potentially curative therapy. However, good QOL for a few months may be of greater value to a patient than a few weeks of hospitalization and potentially fatal transplantation-related toxicity. These considerations frequently do not receive the necessary attention. Published studies typically give figures for disease-free survival, meaning survival without relapse of the disease for which HCT was carried out. Indeed, patients may not be “disease-free” at all. They may have chronic GVHD and chronic pulmonary disease or may show late effects of steroid therapy and other sequelae.94 We are currently not able to predict who will develop chronic GVHD or other complications, but we must inform patients of those possibilities and their effects on QOL; risk averse patients may not want to proceed.
Where do we stand and what do we need?
Over the course of 4 decades, modern HCT has come a long way. Although only 25% of patients have HLA-identical siblings, the development of molecular HLA typing now allows to identify HLA-matched unrelated donors from a pool of more than 10 million for as many as 50% of patients, dependent on ethnicity. Results for many indications are comparable with those achieved with sibling donors. In addition to cells harvested directly from the marrow, stem cells “mobilized” from the marrow into blood can be harvested without the need for anesthesia and may offer accelerated engraftment and enhanced GVT effects. The availability of cord blood cells may allow to further increase the proportion of patients who can be transplanted, and the development of HLA-haploidentical transplantations may make HCT a realistic option for almost any patient.
The creation of multiple conditioning regimens that use different dose intensities and modalities allows us to offer HCT without prohibitive toxicity to patients with some comorbid conditions and to select patients even in their 70s. However, it is important to evaluate the patient's condition in parallel with assessing whether the disease represents an indication for HCT. If we think that HCT, particularly after low-intensity conditioning, is unlikely to provide definitive therapy, as may be the case for acute leukemias resistant to chemotherapy, the patient's fitness for HCT becomes irrelevant.
So who is fit for HCT? Ideally, the patient should have no or only minimal comorbidities. Under those conditions, patients up to 60 and maybe 65 years of age appear to tolerate higher-intensity conditioning as well as younger patients. Of course, as emphasized (Figure 1), one can debate what represents high-dose and what reduced intensity.19 Available data suggest, for example, that a regimen combining fludarabine and busulfan (8 mg/kg) in patients with AML or MDS is associated with success rates (NRM, survival) similar to those seen with cyclophosphamide and busulfan or fludarabine plus busulfan (16 mg/kg) in combination with thymoglobulin.22,95,96 Success rates appear to be lower with further reduction in intensity using, for example, fludarabine combined with 200 cGy of TBI97 ; this particular study involved older patients with prominent comorbidities. In adult patients with ALL, on the other hand, high-dose regimens, such as cyclophosphamide plus 6 × 200 cGy of TBI, appear to result in superior outcome. Of course, to qualify for this dose intensity, no comorbidities should be present, and patients should be no older than 60 years.
Furthermore, the patient's disease should be in remission or should at least have shown responsiveness to pre-HCT therapy. Although there are exceptions, patients with refractory disease pre-HCT often experience progression after HCT when conditioned with low-intensity regimens. Even high-dose regimens may show limited efficacy, including younger patients and patients without comorbidities.
Patients must have a suitable donor, a good social support system, and a secure financial net. They must be well informed, not only about the transplantation process but also about expected or potential post-HCT events, including GVHD and delayed effects, which may become manifest only years after HCT. Treatment with glucocorticoids in particular can have severe side effects in older persons.
We need more data that tell us how a given pre-HCT condition affects post-HCT outcome. Retrospective analyses of pre-HCT findings in patients selected on the basis of presumably identical criteria, but with either successful or unsuccessful post-HCT outcome, may uncover additional factors relevant to the decision-making process. Controlled prospective trials should yield important information in regards to the optimal conditioning regimen for a given disease/risk group, with or without comorbid conditions. Comorbidities, even if assigned the same numerical score, may have different impacts in a 35-year-old than in a 70-year-old patient. The impact of race and socioeconomic status deserves further investigation.
Of course, the field continues to evolve, and the target parameters will change. At this time, we have only limited information as to how DNA polymorphisms affect transplantation outcome. A recent study suggests that Toll-like receptor 4 polymorphism determines the course of aspergillus infections after HCT.98 Ongoing work is investigating whether DNA polymorphisms will allow us to determine the risk of developing GVHD and whether GVHD will respond to steroid therapy. Although not yet within reach, it may be possible to generate a systems biology approach that allows for selection of a given patient with a certain diagnosis for a specific treatment strategy.
Acknowledgments
The authors thank Drs Susan G. Nayfield, William Hazzard, Fred Appelbaum, and Elihu Estey for their comments and constructive suggestions and Helen Crawford and Bonnie Larson for help with manuscript preparation.
This work was supported by the National Institutes of Health, Bethesda, MD (grants P01HL036444, P01CA078902, and P01CA018029).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its subsidiary institutes and centers.
National Institutes of Health
Authorship
Contribution: H.J.D. and B.M.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: H. Joachim Deeg, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: jdeeg@fhcrc.org.