Abstract
Treatment of autoimmune cytopenias remains unsatisfactory for patients refractory to first-line management. We evaluated the safety and efficacy of low-dose rituximab plus alemtuzumab in patients with steroid-refractory autoimmune hemolytic anemia and immune thrombocytopenic purpura. Nineteen of 21 included patients were assessable for response (11 with immune thrombocytopenic purpura, 8 with autoimmune hemolytic anemia). Treatment with 10 mg of alemtuzumab subcutaneously on days 1 to 3, plus 100 mg of rituximab intravenously weekly in 4 doses, was administered. The overall response rate was 100%, with complete response in 58%. The median response duration was 46 weeks (range, 16-89 weeks). Median follow-up was 70 weeks (range, 37-104 weeks). Most toxicity was grade 1 fever related to the first dose. Six patients developed infections. The combination of rituximab and alemtuzumab is feasible and has an acceptable safety profile and remarkable clinical activity in this group of patients. This study is registered at www.clinicaltrials.gov as #NCT00749112.
Introduction
Autoimmune hemolytic anemia (AIHA) and immune thrombocytopenic purpura (ITP) are characterized by antibody-mediated destruction of red blood cells and platelets; as in other autoimmune disorders, B and T lymphocytes have important roles in their pathogenesis.1 Corticosteroids are the standard initial treatment, with a 60% response rate. Patients unresponsive to corticosteroids usually experience a complicated course and show increased morbidity and mortality rates. Splenectomy and a large number of drugs have been used as second-line therapies with variable success, without evidence that any single agent is more effective than another.2
Rituximab effectively depletes B cells involved in the production of antibodies and has been studied as a second-line treatment for ITP at full3-10 and low doses11,12 ; its use in ITP has led to a median overall response rate of approximately 60%.7,9 Alemtuzumab, a humanized IgG monoclonal antibody specific for the CD52 antigen, expressed on lymphocytes, has been used in the treatment of lymphoproliferative disorders and recently for autoimmune diseases.13,14 Preliminary results indicate that alemtuzumab may induce responses in patients with autoimmune cytopenias.15-18
We evaluated the safety and efficacy of low-dose rituximab plus alemtuzumab in combination therapy in patients with steroid-refractory AIHA and ITP. The rationale for combining the 2 monoclonal antibodies was their reported single-agent activity, and the possibility of a synergistic effect, based on the fact that T lymphocytes are involved in the control of expansion of immunoglobulin-producing, autoreactive B-lymphocyte clones.
Methods
Approval for the study was obtained from the Ethics Committee of the School of Medicine and University Hospital of the Universidad Autónoma de Nuevo León. Eligible patients were adults with active symptomatic ITP or AIHA, who had previously received treatment with at least one line of therapy, or followed a chronic relapsing course, and who provided written informed consent. Patients were excluded if they had active bacterial or viral infection, HIV, hepatitis C virus, cytomegalovirus immunoglobulin M (CMV-IgM)–positive serology, hepatitis B surface antigen positivity, pregnancy, or concomitant malignant disease. Pretreatment assessment included complete blood, reticulocyte, lymphocyte subset counts, serum immunoglobulins, direct antiglobulin tests, bilirubin levels, and serum lactic dehydrogenase determinations.
Alemtuzumab was administered at a fixed dose of 10 mg subcutaneously on days 1 to 3, and rituximab at dose of 100 mg intravenously on days 4, 11, 18, and 25; premedication consisted of acetaminophen and chlorpheniramine. All patients received prophylactic trimethoprim/sulfamethoxazole, itraconazole, and acyclovir during and 8 weeks after treatment. Responses were evaluated every week for 1 month, biweekly up to 6 months, and then monthly. Lymphocyte subsets and serum immunoglobulins were assessed at 8 and 24 weeks; CMV antigenemia was determined biweekly during follow-up. Criteria for complete response (CR) for patients with AIHA were normalization of hemoglobin and resolution of hemolysis. A partial response (PR) was defined as a stable increase in the hemoglobin concentration of at least 2.0 g/dL, resolution of hemolysis, and transfusion independence. CR and PR in patients with ITP were defined as a platelet count ≥ 150 × 109/L and ≥ 50 × 109/L, respectively, on 2 consecutive occasions.
Results and discussion
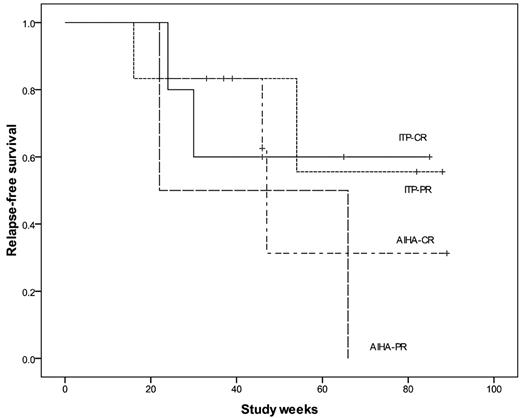
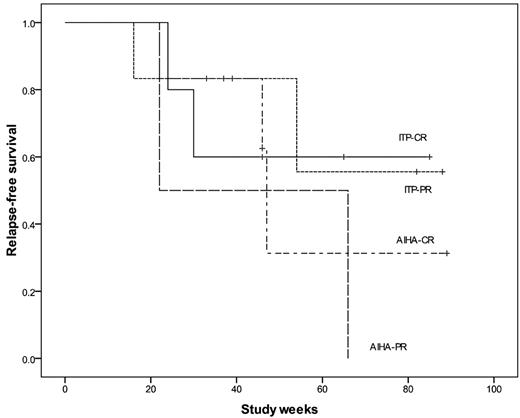
From August 2008 to January 2010, 21 consecutive adult patients were included; 19 completed the therapeutic schedule with a median follow-up of 70 weeks (range, 37-104 weeks). One patient died and another was lost to follow-up after 2 weeks of therapy. Of the 11 patients with ITP (median age, 26 years; range, 16-71 years), the median duration of ITP was 4 years, and the median number of prior treatments was 2.5 (Table 1). Five patients achieved CR: 2 of them maintained it up to the last visit, 1 patient lost CR but remains in PR, and 2 patients relapsed. Six of the 11 patients achieved a PR: 2 relapsed, 4 patients maintained PR, with no additional treatment in 2, and 2 patients are receiving danazol 100 mg/d. The median duration of CR was 46 weeks (range, 16-88 weeks; Figure 1).
Relapse-free survival in ITP and AIHA patients after low-dose rituximab and alemtuzumab. Relapse-free survival was considered as the period of maintenance of the best response achieved (ie, CR or PR).
Relapse-free survival in ITP and AIHA patients after low-dose rituximab and alemtuzumab. Relapse-free survival was considered as the period of maintenance of the best response achieved (ie, CR or PR).
All 11 patients with ITP achieved a response at 1 week; these early responses have been described, suggesting an additional mechanism of action of rituximab through saturation of Fc receptors of the monocyte-macrophage system by opsonized B cells.19-21 After therapy with the antibodies, corticosteroids could be discontinued in all 11 patients. Worsening thrombocytopenia has been previously reported in patients with ITP treated with alemtuzumab,17 but we did not observe it. This response rate was higher than that observed with any single agent.4-12,15-18
All 8 patients with AIHA had the warm antibody form (median age, 55 years; range, 26-71 years). Responses were observed in all patients: 6 achieved CR, 3 of them are still in CR, and 3 patients relapsed. The 2 patients who achieved PR relapsed. The median duration of CR was 46 weeks (range, 22-89 weeks).
The experience with the use of alemtuzumab in patients with autoimmune cytopenias has been very limited. Small series of patients indicate that alemtuzumab at conventional doses15-18 may induce responses in severe AIHA cases, and lower doses have been also effective in other immunologic diseases.22,23 In our study, 3 of 8 patients with AIHA maintained CR at 33, 46, and 89 weeks. One relapsing patient with AIHA achieved CR after a second course of therapy; it is worth noting that we used a low dose and short course of monoclonal antibodies, but future studies should explore the possibility of using some kind of maintenance or increasing the dose and/or duration of initial treatment.
Therapy was well tolerated in all patients, apart from first-day reactions. Grade 1 fever was observed in 90% of patients after alemtuzumab administration; no adverse events were reported with the rituximab infusion. One patient with ITP died in her home during the third week of therapy; she had a rising platelet count, without any signs of bleeding or infection. Two patients had herpes zoster reactivation, 2 had urinary tract infection, and 2 additional patients had upper airway infection; all of them had a favorable outcome after receiving oral antiviral/antibiotic therapy. CMV infection was not detected in any of our patients; this may be related to the lower alemtuzumab dose and prophylactic acyclovir used in this study. Others have also studied this combination at conventional doses in patients with lymphoid malignancies, with an acceptable safety profile.24,25
Alemtuzumab induces profound lymphopenia; B cells and NK cells recover after 4 to 6 weeks, but T cells reach normal levels at a slower rate.10,15,18 In our study group, CD4+ T cells remained at low levels at week 24, whereas B and NK cells partially recovered to basal levels. All patients had normal IgG and IgM basal levels, without significant changes at 8 and 24 weeks of treatment.
This is the first trial to combine rituximab and alemtuzumab in patients with AIHA and ITP. We think that the results obtained are related mainly to the antibody combination. Potential advantages of using monoclonal antibodies at lower doses include the avoidance of severe side effects, steroid-sparing effects, splenectomy-sparing capacity, and the possibility to administer in repeated courses. The strengths of this study are the prospective methodology, the high overall response rate, and the prompt response achieved in patients with ITP. Limitations included the short follow-up, small sample size, and the lack of a control group.
In conclusion, low-dose rituximab and alemtuzumab in combination therapy is a therapeutic option for ITP and selected cases of AIHA and may be an effective alternative to splenectomy and other immunosuppressive agents.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nereida Méndez and Rosario Salazar for technical assistance.
Authorship
Contribution: D.G.-A. designed the research and wrote the paper; M.S.-G. performed research and analyzed data; L.T.-A. analyzed data and wrote the paper; J.L.H.-G. analyzed data; O.G.C.-R. and C.H.G.-A. performed research; and J.C.J.-P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Gómez-Almaguer, Hematology Service, Hospital Universitario, “Dr José E. González,” Universidad Autonoma de Nuevo Leon, Av Madero y Gonzalitos S/N, Col Mitras Centro, Monterrey, NL CP 64460, México; e-mail: dr_gomez@infosel.net.mx.