Abstract
To identify prognostic factors for patients transplanted for relapsed or refractory Hodgkin lymphoma we carried out a combined analysis of patients followed prospectively on 3 consecutive protocols at Memorial Sloan-Kettering Cancer Center. One hundred fifty-three patients with chemosensitive disease after ICE (ifosfamide, carboplatin, and etoposide)–based salvage therapy (ST) proceeded to high-dose chemoradiotherapy followed by autologous stem cell transplantation (ASCT). Patients were evaluated with computed tomography and functional imaging (gallium or fluorodeoxyglucose-positron emission tomography) prior to ST and again before ASCT. Functional imaging status before ASCT was the only factor significant for event-free survival (EFS) and overall survival by multivariate analysis and clearly identifies poor risk patients (5-year EFS 31% and 75% for FI-positive and negative patients respectively). Administration of involved-field radiotherapy with ASCT was marginally significant for EFS (P = .055). Studies evaluating novel STs, conditioning regimens, post-ASCT maintenance, or allogeneic stem cell transplantation are warranted for patients who fail to normalize pre-ASCT functional imaging.
Introduction
The standard treatment for relapsed or refractory (rel/ref) Hodgkin lymphoma (HL) is high-dose chemoradiotherapy followed by autologous stem cell transplantation (ASCT)1,2 ; this treatment cures approximately 50% of patients.2-4 Eligibility for ASCT is based upon the presence of chemosensitive disease to salvage therapy (ST) on computed tomography (CT). While many patients are evaluated by both CT and fluorodeoxyglucose-positron emission tomography (FDG-PET) prior to ASCT, it is currently standard practice for FDG-PET–positive patients to proceed to transplantation as long as they show evidence of response to ST on CT. FDG-PET is now an integral component of the response assessment for HL, and it has been shown to be prognostic at interim analysis during front-line treatment5,6 as well as prior to ASCT.7-12 Since 1994, patients with rel/ref HL presenting to our institution were treated on 1 of 3 consecutive studies and received ICE (ifosfamide, carboplatin, etoposide)–based ST followed by ASCT. All patients were evaluated before ST and ASCT with CT and functional imaging (FDG-PET or gallium). We aimed to determine the prognostic impact of functional imaging (FI) and other factors on outcome following ASCT in this group, which represents the largest series of patients receiving ICE-based ST before ASCT.
Methods
Patients
Consecutive patients at Memorial Sloan-Kettering Cancer Center (MSKCC) treated for rel/ref HL from 1994 to December 2003 were identified. Rel/ref disease was confirmed by biopsy. A waiver of authorization to carry out this analysis was approved by the institutional review board (IRB) at MSKCC.
ST
All patients received ICE-based ST followed by ASCT on 1 of 3 consecutive protocols or as per protocol. Treatment details were previously described.3,13 In brief, patients treated from 1994 to 1998 received 2 cycles of standard ICE and patients treated from 1998 to 2003 received risk- stratified ST based upon the number of pre-ST risk factors (RFs; B symptoms, extranodal disease, and/or remission duration less than 1 year) present. Patients with 0-1 RF received standard-ICE, those with 2 RFs received 1 cycle of standard ICE and 1 cycle of augmented ICE, and those with 3 RFs received high-dose ICE with autologous stem cell rescue. Patients were evaluated by FI (either gallium or FDG-PET) and CT prior to ST and prior to ASCT; those with at least minimal response to ST proceeded to ASCT.
Conditioning regimens
Conditioning consisted of total lymphoid irradiation plus high-dose cyclophosphamide and etoposide for radiation-naive patients or CBV (cyclophosphamide, BCNU, and etoposide) for those previously irradiated. Involved field radiation therapy (IFRT) was administered prior to ASCT to all sites of residual or relapsed nodal-based disease that were not previously irradiated. Patients received up to 3600 cGy radiation (combined IFRT and total lymphoid irradiation) administered in 20 fractions over 2 weeks prior to high-dose chemotherapy.
Statistical analysis
Event-free survival (EFS) and overall survival (OS) were calculated from the time of ST using the Kaplan-Meier method. Primary refractory disease was defined as failure to achieve a complete remission following first-line treatment, documented by biopsy. Both univariate and multivariate analysis were performed. For multivariate analysis, only significant factors were retained in the final model. P values ≤ .05 were considered significant. Hazard ratios (HRs) were estimated from Cox regression models. The Pearson χ2 test was used to evaluate for an association between type of imaging and site of disease.
Results and discussion
Between 1994 and 2003, 189 patients received ST at MSKCC for relapsed or refractory HL; 153 (81%) patients proceeded to ASCT and represent the focus of this analysis. The characteristics of the 153 patients appear in Table 1. One hundred thirty-six patients were treated on IRB-approved protocols; 17 patients were treated off study due to no available study at the time of their presentation. Forty-three (28%) patients had positive FI prior to ASCT.
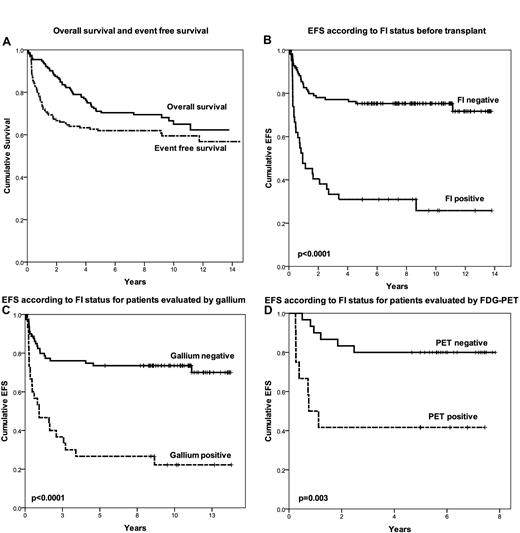
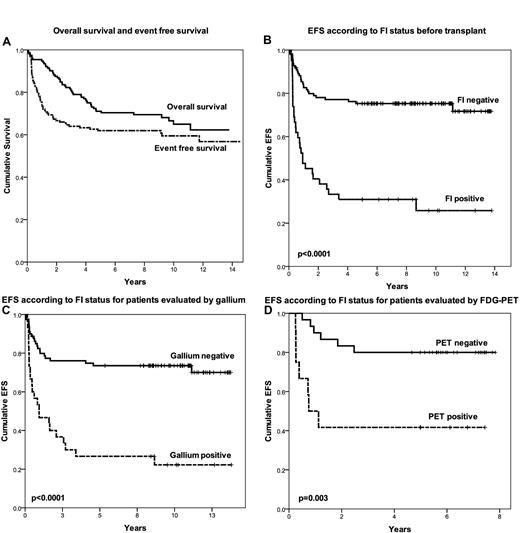
Figure 1A displays the EFS and OS for the entire group. Survivors were followed for a median of 8.6 years. The 5-year EFS and OS were 63% and 71%, respectively. The pre-salvage factors significant for EFS in univariate analysis were bulky nodal disease (≥ 5 cm), extranodal disease, FI normalization, normalization of CT, and administration of IFRT (at time of transplantation). Factors significant for OS in univariate analysis were bulk, extranodal disease, and FI normalization. The only factor that remained significant in multivariate analysis for both OS and EFS was normalization of FI (HRs 3.37 and 3.74 for EFS and OS, respectively). Figure 1B shows the Kaplan-Meier curve for EFS based upon FI status prior to transplantation. The 5-year EFS for FI-negative and FI-positive patients were 75% and 31%, respectively (P < .0001). Imaging by both gallium and FDG-PET was strongly predictive for outcome (Figure 1C-D), and there was no difference in outcome for patients evaluated by FDG-PET or gallium (P = .3), thus providing rationale for analyzing the results from gallium and FDG-PET together. Administration of pre-ASCT IFRT was associated with a marginally significant benefit for EFS in multivariate analysis (P = .055) and this benefit was seen for patients analyzed by either gallium or FDG-PET (P = .05 and .04, respectively). Detailed long-term toxicity associated with high-dose chemotherapy and radiation at our institution was previously reported.14
Patient outcome. (A) OS and EFS. (B) EFS according to FI status before transplantation. (C) EFS according to FI status for patients evaluated by gallium. (D) EFS according to FI status for patients evaluated by FDG-PET.
Patient outcome. (A) OS and EFS. (B) EFS according to FI status before transplantation. (C) EFS according to FI status for patients evaluated by gallium. (D) EFS according to FI status for patients evaluated by FDG-PET.
This analysis represents the largest series of patients followed prospectively on IRB approved trials and treated with uniform ST. Consistent with other reports, the outcome for FI-positive patients is poor with median EFS of 11 months and 5-year EFS of 31%. The majority of patients in our series were evaluated by gallium scans; however, both gallium and FDG-PET were strongly predictive for outcome. Patients evaluated by FDG-PET were more likely to have abnormalities in bone (21% vs 7%, P = .013) or muscle (14% vs 3.6%, P = .018); however, the significance of this is unclear since bone abnormalities can persist despite disease response and muscle uptake could represent benign physiologic activity. Although FDG-PET has mostly replaced gallium scans for evaluation of lymphoma, these data suggest that while gallium may not be as sensitive as FDG-PET in detecting disease in certain sites, it remains a valuable tool for assessing patients in the pre-ASCT setting, particularly in centers where FDG-PET is not available or cost prohibitive.
Approximately one-third of patients with abnormal pre-ASCT FI are cured with ASCT, and therefore, ASCT remains the standard treatment. However, a portion of these patients may be better served by other treatment strategies. Patients with abnormal pre-ASCT FI should be enrolled on prospective studies evaluating novel salvage regimens, new conditioning regimens, or posttransplantation maintenance therapies. Administration of IFRT was an independent predictor for EFS; however, the true role of IFRT in this setting is unclear because patients were selected for IFRT based upon disease distribution and treatment history. Among 115 patients who received IFRT, 31 ultimately relapsed and 19 (17%) relapsed within radiation fields. Sites of relapse (nodal versus extranodal) did not differ significantly between IFRT and non-IFRT patients; however, a trend toward fewer nodal-only relapses was seen among the IFRT patients, particularly among the FI-positive patients (41% vs 75% nodal-only relapses), suggesting that IFRT reduced the rate of relapse in irradiated sites. Patients who remain FI-positive and are ineligible for IFRT represent a particularly poor-risk group; only 2 of 12 of these patients in our series are event-free after transplantation. These poor-risk patients are optimal candidates for prospective studies evaluating allogeneic stem cell transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Lymphoma Foundation and the Singer Family Lymphoma Research Fund.
Authorship
Contribution: A.J.M. designed and performed research, analyzed data, and wrote the manuscript; J.Y. designed and performed research; T.K. designed and performed research; J.C.M. performed research and analyzed data; J.M.V. performed research and analyzed data; A.D.Z. designed and performed research and analyzed data; and C.H.M. designed and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alison J. Moskowitz, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: moskowia@mskcc.org.