Abstract
Proapoptotic Bax and Bak are the key B-cell lymphoma-2 family members mediating apoptosis through the intrinsic pathway. Cells doubly deficient for Bax and Bak are profoundly resistant to apoptotic stimuli originating from multiple stimuli. Here we describe mice in which Bax and Bak have been deleted specifically in T-cells using Lck-Cre. In these T cell–specific BaxBak-deficient mice, early T-cell progenitors accumulate in the thymus, with relative depletion of more mature T cells. In addition, bone marrow progenitor cells fail to progress to the double positive stage when cultured on OP9 stromal cells expressing the Notch ligand Delta-like 1, consistent with a critical role for Bax and Bak in early T-cell development. Over time, T cell–specific BaxBak-deficient mice progress to an aggressive T-cell lymphoblastic leukemia/lymphoma. Interestingly, quantitative real-time polymerase chain reaction analysis of BaxBak-deficient T-cell lymphomas does not display amplification of the Notch signal transduction pathway, commonly activated in T-cell leukemia in both mouse and man. Bax and Bak, key regulators of the intrinsic pathway of apoptosis, are thus required to prevent T-cell malignancy, and for normal T-cell differentiation, regulating early T-cell development at the stage of early T-lineage progenitor cells.
Introduction
The B-cell lymphoma (Bcl)–2 family of proteins constitutes a critical checkpoint upstream of irreversible cell damage. The founding family member, Bcl-2 was cloned at the t (14:18) chromosomal breakpoint, the molecular hallmark of follicular B-cell lymphoma, establishing an important role for this family in regulating hematopoietic tumorigenesis.1-3 Both pro and antiapoptotic family members have been identified. The proapoptotic members can be further subdivided into “multidomain” members, such as Bax and Bak, harboring 4 Bcl-2 homology (BH) domains (BH1-4), and BH3-only members such as Bid, Bad, Bim, Bik, Noxa, and Puma. Mouse embryonic fibroblasts in which both Bax and Bak have been deleted are profoundly resistant to multiple apoptotic stimuli, indicating that these proteins comprise a gateway to apoptosis through the mitochondria.4
Apoptosis plays an important role in the immune system to regulate homeostasis after antigen-induced cellular fluxes as well as to cull cells that fail proper differentiation. T cells rely on apoptosis to craft the enormous diversity that allows an effective response to immune challenge but prevents inappropriate autoimmunity. During development, T cells proceed through a series of apoptotic “checkpoints” including beta selection as well as positive and negative selection to cull those cells harboring nonfunctional or auto reactive T-cell receptor (TCR) complexes. When over expressed in a TCR transgenic mouse system encoding reactivity for the male antigen HY presented by the H-2Db class I antigen of the major histocompatibility complex (MHC),5 antiapoptotic Bcl-2 inhibits the death of nonselectable thymocytes and reduces the deletion of self-reactive thymocytes in male mice, but does not inhibit negative selection by superantigen.6
Mice in which both Bax and Bak are deleted in the germ line demonstrate multiple developmental defects and reduced viability.7 Reconstitution of Rag−/− mice with BaxBak doubly deficient BM reveals defects in selection of thymocytes and altered representation of thymic subtypes.8 In addition, BaxBak doubly deficient T cells display defective death by neglect as well as defective antigen receptor-induced apoptosis.8 Despite the crucial role of apoptosis in crafting antigen-specific T cells, BaxBak deficient thymocytes manifest only a modest expansion. Interestingly, these thymocytes accumulate progenitor cells before the apoptotic checkpoints of positive and negative selection, with a relative depletion of more mature T cells. These mature T cells display defective activation-induced T-cell proliferation due to decreased endoplasmic reticular (ER) calcium stores in the absence of Bax and Bak.9 Thus, Bax and Bak are required for normal thymic selection and T-cell activation.
Although the role of apoptosis in post beta selection phases of T-cell development has been extensively studied, the role of apoptosis in early T-cell development is not well understood. Early T-cell development in the thymus proceeds through a series of highly ordered and regulated stages that are defined at least in part by cell surface markers. T cells progress from cluster of differentiation (CD)4−CD8−double negative (DN) cells, to CD4+CD8+ double positive (DP) cells, to single positive (SP) CD4+ or CD8+ T cells. The DN stage can be further subdivided based on CD25 and CD44. The early hematopoietic progenitors that give rise to the T lineage, the early T-cell lineage progenitors (ETPs)10 migrate from the BM before definitive T-cell lineage commitment,11 and possess multipotent differentiative capacity. These ETP cells comprise a subset of cells within the CD3−CD4−/lowCD8−CD25−CD44highc-kithigh or DN1c-kithigh fraction.12 At the second or DN2 stage, T-cell progenitors down-regulate the expression of c-kit (CD4−CD8−CD25+CD44+). T cells irreversibly commit to the T-cell lineage upon pre-TCR rearrangement at the DN3 stage (CD4−CD8−CD25+CD44−).13
We used mice in which Bax is conditionally deleted in the T-cell lineage using LckCre on a Bak−/− background (LckCre+BaxBakDKO) to study the effect of BaxBak deficiency on T-cell development in the context of an otherwise normal mouse, and to evaluate the long-term consequences of the abnormal BaxBak-deficient T-cell differentiation. Despite a pronounced defect in thymocyte apoptosis when treated with multiple stimuli in vitro, we find only a modest increase in thymus size. In addition, we find an increase in the early thymic progenitors, but a decrease in thymic progenitors from the DN2 stage onward, suggesting a block in differentiation in unstressed LckCre+BaxBakDKO mice. Furthermore, BaxBak-deficient BM progenitor cells fail to differentiate along the T lineage in the OP9-DL1 in vitro differentiation system,14 consistent with an early defect in thymocyte differentiation in absence of Bax and Bak. Over time, these mice doubly deficient for Bax and Bak develop an aggressive T-cell leukemia/lymphoma. Surprisingly, although Notch activation is seen in nearly all mouse models of T-cell leukemia, and 50% of tumors in human T-cell acute lymphoblastic leukemia (T-ALL),15 Notch1 is not up-regulated in these BaxBak DKO T-cell malignancies. Thus we demonstrate an important role for Bax and Bak in early T-cell differentiation and prevention of malignancy
Methods
Mice
The Bax and Bak double deficient (DKO) mice were generated as described previously.16 Briefly, exons 2-4 (Figure 1A) of Bax flanked by LoxP sites were inserted along with a neo gene.16 After electroporation into RW4 embryonic stem (ES) cells, clones resistant to Geneticin reagent (G418) were screened for homologous recombination. ES cells were transfected with cytomegalovirus-Cre to excise the Neo gene. Successfully recombined ES cells were injected into C57Bl/6 blastocysts. The mice were further crossed with Bak−/− mice. Conditional deletion of Bax was obtained by crossing mice with transgenic mice expressing Cre recombinase driven by either the Lck promoter or the interferon responsive Mx-1 promoter. In the case of Mx-Cre mice, Cre recombinase was induced by injection with poly (I: C), 30 μg intraperitoneally every other day ×3 (Invivogen). Mice were bred and maintained in the animal facility of Vanderbilt University Medical Center in specific pathogen-free conditions, and all animal protocols were reviewed and approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Flow cytometry
Phycoerithrin (PE)–, fluorescein isothiocyanate (FITC)–, Peridinin chlorophyll protein (PerCP-Cy-5.5)–, or allophycocyanin (APC)–conjugated anti–mouse monoclonal antibodies against CD4, CD8α, CD44, CD25, C-kit, TCRβ, and TCRγδ were purchased from BD Biosciences. Flow cytometry was performed on a FACSCaliber or BD LSR II (BD Biosciences). Data analysis was performed using FlowJo Version 6.4 analysis software (TreeStar).
Analysis of T-cell rearrangement
Genomic DNA was prepared from thymocytes obtained from Lck–Cre–positive and control mice using phenol chloroform (Sigma-Aldrich). Ten nanograms of DNA was amplified using TCR Dβ2: TGAGAGCTGTCTCCTACTATCGATT and Jβ2 locus specific primers: ATTGTGGGGACTGGGGGGGC with 52°C annealing temperature, and separated by electrophoresis on a 1% agarose gel in Tris [tris(hydroxymethyl)aminomethane] borate buffer.
Cell sorting
Thymocytes (100 million) were sorted after staining with CD4 (FITC), CD8 (FITC), CD25 (PerCP-Cy5.5), and CD44 (PE) at a cell density of 107 cells/mL at a pressure of 70 μm in 3% fetal bovine serum (FBS) at the Flow Cytometry Core at Vanderbilt University Medical Center.
Detection of Bax in thymocytes by immunofluorescence
Thymocytes were isolated from control or BaxBak DKO mice, and fixed in 4% paraformaldehyde for 20 minutes. After washing with phosphate-buffered saline (PBS), pellets were resuspended in 70% ethanol and embedded in paraffin for cellblocks. Cell slides were heated at 59°C for 1 hour, deparaffinized with xylene, and hydrated with a graded alcohol series. Vector M.O.M. immunodetection kit was used for this fluorescein staining. A 1:200 dilution of the anti-Bax clone 6A7 (Calbiochem) was used for primary antibody and 1:1000 dilution of biotinylated anti–mouse for secondary antibody (Vector M.O.M. immunodetection kit). Fluorescein Avidin DCS (M.O.M. kit) was used for color detection. Samples were visualized on a Leica DM IRB inverted immunofluorescence microscope (Vanderbilt University Medical Center cell imaging shared resource).
Intracellular staining of Bax
To determine the expression of Bax in various subpopulations of thymocytes, the cells were stained with CD4, CD8, CD25, CD44, and ckit-specific antibodies. After surface staining, the cells were fixed with Cytofix/Cytoperm (BD Biosciences), the cell suspensions were permeabilized with BD Cytoperm Plus. The cells were then incubated in saponin buffer (PBS containing 3% FBS and 0.3% saponin (Sigma-Aldrich) for 15 minutes, and blocked with mouse immunoglobulin G (IgG) blocking solution for 30 minutes. After washing with saponin buffer, cells were incubated with purified monoclonal anti–mouse Bax for 30 minutes. Purified IgG1 (EMD Biosciences) was used as an isotype-matched control. Cells were washed with saponin buffer, incubated with goat anti–mouse IgG Alexa Fluor 488 conjugated secondary antibody (Invitrogen) for another 20 minutes, and washed with PBS.
Purification of BM progenitor cells
BM was enriched for precursor cells by lineage depletion. Briefly, cells were incubated with biotinylated anti-lineage antibodies (lin = anti-CD3, B220, Gr1, Ter119 [Pharmingen]), washed, and incubated with streptavidin conjugated magnetic beads (Dynal). Lineage positive cells were depleted by magnetic sorting. Lineage-depleted BM cells were stained for flow cytometric analysis and in vitro culture.
In vitro T-cell differentiation culture
OP9-DL1 and OP9-control cells were generated from the OP9 BM stromal cell line as described.14 Monolayers of OP9 cells were cultured in OP9 medium (α-MEM supplemented with 20% FBS and 2.2 g/L of sodium carbonate). Mx-Cre+BaxBak DKO and control lineage-depleted BM cells (5 × 103) were induced to differentiate by culture on either OP9-control or OP9-DL1 cell monolayers (60%-70% confluency) in 24-well plates in the presence of exogenously supplied fms-like tyrosine kinase receptor-3 ligand (5 ng/mL) and IL7 (5 ng/mL; R&D Systems). Every 7 days the nonadherent BM-derived hematopoietic cells were collected by pipetting and replated on fresh OP9 monolayers in OP9 media. Cells were removed at each time point for analysis by flow cytometry, and isolation of genomic DNA and RNA.
Cell viability and apoptosis assays
Cell suspensions of thymocytes and splenocytes were cultured in vitro in complete RPMI-with 10% fetal calf serum. Cells were treated with 1mM hydroxyurea (HU), 2 Gy ionizing radiation (IR), or 10 J ultraviolet light (UV). Cell viability was determined by annexin V staining and propidium iodine exclusion followed by flow cytometric analysis.
Real-time quantitative polymerase chain reaction analysis
Total RNA was isolated and purified from lymph nodes of wild type, tumor-containing DKO, and healthy DKO mice using RNeasy Mini Kit (QIAGEN). cDNA was synthesized using Superscript reverse transcriptase (Invitrogen) and oligo-dT primer following the manufacturer's instructions. MRNAs were quantified by quantitative real-time polymerase chain reaction (PCR) with an iCycler (Bio-Rad) and Taq polymerase containing SYBR Green I (Sigma-Aldrich). The sequences of the specific PCR primers were as follows (5′-3′): CreFW: TCCATATTGGCAGAACGAAA; CreRV: CAGCTACA-CCAGAGACGGAA; BaxFW:ACAGATCATGAAGACAGGGG;BaxRV:CAAAGTAGAAGAGGGCAACC DeltexFW: ATCAGTTCCGGCAAGACACAG; DeltexRV: CGATGAGAGGTC GAGCCAC; Hes-1FW: CCAGCCAGTGTCAACACGA; Hes-1RV:AATGCCGG GAGCTATCTTTCT; Notch-1FW: TGGACATCCGTGGCTCCATTGTCT; Notch-1RV: TCCGCTTCTTCTTGCTGGCCTCTG. Each primer pair was also tested with a logarithmic dilution of a cDNA mix to get a linear curve.
Results
Mice harboring conditional deletion of Bax and Bak in thymocytes display abnormal thymocyte development
Proapoptotic Bax and Bak are key mediators of the intrinsic pathway at the mitochondria. Mice deficient for both Bax and Bak display developmental and homeostatic irregularities in multiple tissues including the hematopoietic system.7 To investigate the impact of Bax and Bak deletion on T-cell differentiation, we have bred the LckCre transgenic mouse into Bak−/− mice harboring a floxed Bax allele (Figure 1A; LckCre+BaxBakDKO mice). Despite the profound defect in apoptosis conferred by Bax and Bak deficiency, LckCre+BaxBakDKO mice demonstrate a modestly enlarged thymus relative to control mice (Figure 1B), consistent with thymocyte numbers observed using BaxBak DKO BM cells transferred into Rag−/− mice.8 Thymic subsets of these mice manifest aberrant T-cell differentiation, with a decrease in DP cells and a concomitant increase in DN and SP populations (Figure 1C-D). Evaluation of the DN populations reveals an increase in the DN1 population with concomitant decrease in the DN3 population (Figure 1E-F and Table 1). Interestingly, there is a profound increase in the C-kit high DN1 subpopulation in the absence of Bax and Bak (Figure 1G-H), representing expansion of a population of early T progenitor (ETP) cells. This suggests either decrease in apoptosis of ETP cells, increased proliferation of ETP cells, or a defective ability of the cells to progress in differentiation from the ETP to the DN2 stage in the absence of Bax and Bak.
Phenotype of thymocyte development in tissue specific knockout of Bax and Bak using Cre/LoxP system. (A) Schematic representation of genomic organization of conditional knockout of Bax. (B) Number of thymocytes in wild-type and DKO mice (mean and SD; 5 animals per group). (C) FACScan (BD Biosciences) analysis of thymic subsets in both healthy and tumor containing LckCre+BaxBak DKO mice using α-CD4 and α-CD8 antibodies. (D) Absolute number of T-cell populations, DN, DP, and SP CD4+ and CD8+ in control and LckCre+BaxBak DKO mice. *P < .05, and **P < .001 (E) FACScan analysis of double negative populations from Cre− (control) and Cre+ thymocytes are subdivided into DN1,DN2, and DN3 based on CD44 and CD25 staining. (F) The absolute numbers of T-cell populations in panel E. (G) Analysis of CD44+c-kithigh subsets (DN1) populations. (H) Absolute numbers of DN1c-kithigh, DN2, and DN3 populations.
Phenotype of thymocyte development in tissue specific knockout of Bax and Bak using Cre/LoxP system. (A) Schematic representation of genomic organization of conditional knockout of Bax. (B) Number of thymocytes in wild-type and DKO mice (mean and SD; 5 animals per group). (C) FACScan (BD Biosciences) analysis of thymic subsets in both healthy and tumor containing LckCre+BaxBak DKO mice using α-CD4 and α-CD8 antibodies. (D) Absolute number of T-cell populations, DN, DP, and SP CD4+ and CD8+ in control and LckCre+BaxBak DKO mice. *P < .05, and **P < .001 (E) FACScan analysis of double negative populations from Cre− (control) and Cre+ thymocytes are subdivided into DN1,DN2, and DN3 based on CD44 and CD25 staining. (F) The absolute numbers of T-cell populations in panel E. (G) Analysis of CD44+c-kithigh subsets (DN1) populations. (H) Absolute numbers of DN1c-kithigh, DN2, and DN3 populations.
Early T-cell progenitors proliferate normally in the absence of Bax and Bak
To determine whether the accumulation of early thymic progenitors is due to increased proliferation of the DN1 population, we evaluated proliferation of these cells in vivo by bromodeoxyuridine (BrDU) incorporation. Control (LckCre-BaxF/+Bak−/−) and LckCre+BaxBakDKO mice were injected intraperitoneally with BrDU, killed after 3 hours, and thymocytes were harvested. Control and LckCre+BaxBakDKO thymocytes incorporated similar amounts of BrDU (Figure 2A), indicating that the increase in the DN1 population in LckCre+BaxBakDKO mice cannot be attributed to increased proliferation of this population of cells in the absence of Bax and Bak.
Abnormal thymocyte development in the absence of Bax and Bak occurs without affecting proliferation and TCRβ rearrangement. (A) To determine the proliferation of premalignant thymocytes, Control and LckCre+BaxBak DKO mice were injected with BrDU intraperitoneally. Mice were killed after 3 hours and the DN CD44+ population of thymocytes was evaluated by immunostaining followed by flow cytometry. (B) Surface TCR-β staining in DN, DP, and SP CD4+ and CD8+ cells. (C) To define the status of β chain rearrangement in the subpopulation of DN thymocyte cells DN1, DN2, DN3, and DN4 (CD44−CD25−) populations were isolated by FACSAria (BD BioSciences) cell sorting. Genomic DNA was isolated from these subpopulations and analyzed by PCR for rearrangements at the TCRβ locus using Dβ2 and Jβ2 region specific primers. PCR products were analyzed on 1% agarose gels. Shown are images of 2 agarose gels with DNA markers on each gel. (D) DN1 and DN3 populations were further specified with CD44+c-kithigh and CD25+c-kitlow gates, respectively and the presence of surface TCR-β was evaluated by flow cytometry.
Abnormal thymocyte development in the absence of Bax and Bak occurs without affecting proliferation and TCRβ rearrangement. (A) To determine the proliferation of premalignant thymocytes, Control and LckCre+BaxBak DKO mice were injected with BrDU intraperitoneally. Mice were killed after 3 hours and the DN CD44+ population of thymocytes was evaluated by immunostaining followed by flow cytometry. (B) Surface TCR-β staining in DN, DP, and SP CD4+ and CD8+ cells. (C) To define the status of β chain rearrangement in the subpopulation of DN thymocyte cells DN1, DN2, DN3, and DN4 (CD44−CD25−) populations were isolated by FACSAria (BD BioSciences) cell sorting. Genomic DNA was isolated from these subpopulations and analyzed by PCR for rearrangements at the TCRβ locus using Dβ2 and Jβ2 region specific primers. PCR products were analyzed on 1% agarose gels. Shown are images of 2 agarose gels with DNA markers on each gel. (D) DN1 and DN3 populations were further specified with CD44+c-kithigh and CD25+c-kitlow gates, respectively and the presence of surface TCR-β was evaluated by flow cytometry.
β chain rearrangement occurs normally in the absence of Bax and Bak in T-cell subsets in vivo
To further investigate the origin of the DN cells observed in Lck-Cre+BaxBakDKO thymocytes, we evaluated the status of beta chain rearrangement in various T-cell populations from control and Lck-Cre+BaxBakDKO mice. Lck-Cre+BaxBakDKO cells that progress from DN to the DP stage and subsequent SP CD4+ and CD8+ cells demonstrate normal expression of TCR-β (Figure 2B). DN3 cells that have generated a functional TCRβ chain, which can pair with the invariant pre-Tα and the CD3 signaling chains to form a pre-TCR to promote maturation to the next differentiation stage (DN4).13 Cells that fail to generate a functional TCRβ chain are removed by apoptosis, and this β selection represents one of the first checkpoints during T-cell development. As noted in the previous paragraph, a defect in cell death due to negative selection could potentially result in cells post beta chain rearrangement that lack surface TCR and would thus appear to be DN cells. To further define the status of β chain rearrangement in LckCre+BaxBak DKO T-cell progenitor populations, we isolated cells in each of the DN populations by flow sorting. PCR analysis was performed to determine the Dβ2 to Jβ2 rearrangements on genomic DNA isolated from DN1, DN2, DN3, and DN4 (CD44−CD25−) stages of LckCre+BaxBak DKO and control thymocytes. In control thymocytes, β chain rearrangement is first evident at the DN3 stage and persists to the DN4 (CD44−CD25−) populations (Figure 2C). In the absence of Bax and Bak, the DN1 population demonstrates a predominant PCR product from the genomic beta chain locus, consistent with cells that are pre-beta chain selection. Of note, shorter PCR products are evident, consistent with the presence of some cells that are post beta chain selection. To further define beta chain rearrangement at the cellular level, we evaluated cells in the C-kit high population of DN1 cells for surface TCRβ staining by flow cytometry. Neither Lck-Cre+BaxBakDKO or control DN1 C-kit high cells demonstrate TCRβ expression, consistent with pre-beta chain rearranged cells. LckCre+BaxBakDKO DN3 cells are greatly reduced in number, but display normal beta chain rearrangement (Figure 2C) with normal low TCR β surface expression in the absence Bax and Bak (Figure 2 D).
Rathmell et al8 have described an expanded CD44int DN population that displays evidence of TCR rearrangement by CD3 staining. They attribute the expansion of this population to cells that have failed thymic selection but were not eliminated due to defective apoptosis in the absence of Bax and Bak. We also note this population in our LckCre+BaxBakDKO mice, and similarly find that these cells are CD3+ (supplemental Figure 1B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Our results are thus consistent with those reported by Rathmell et al.8 In contrast, the DN1 population that is expanded in LckCre+BaxBakDKO mice, as defined by CD4−CD8−CD44+c-kithigh cells does not demonstrate increased CD3 staining, consistent with a population that has not undergone TCR rearrangement (supplemental Figure 1A,C).
LckCre+BaxBak DKO mice delete Bax in DN1cKithigh thymocytes
Taken together our results suggest a defect in early T-cell development, at the stage of the early T-lymphocyte progenitor. To determine whether this phenotype correlates with the deletion of Bax, we evaluated thymi from control and LckCre+BaxBakDKO mice for Bax expression by immunofluorescence. Our immunofluorescence data show the absence of Bax in Lck-Cre+BaxBak DKO thymocytes (Figure 3A). Furthermore, real-time PCR analysis of sorted progenitor cell populations demonstrates evidence of Cre expression in the ETP population (Figure 3B). To further define deletion of Bax at the cellular level, we evaluated cells in the DN1c-kithigh population of ETP cells for intracellular Bax by flow cytometry (Figure 3C-D). There is a profoundly expanded population of DN1c-kithigh cells in the ETP population in the absence of intracellular Bax (Figure 3A). Overall, the efficiency of Bax deletion in the total thymocyte population of our LckCre+BaxBak DKO mice is 86.6% ± 2.62% (supplemental Figure 1D). Thus in our model, LckCre drives deletion of Bax as early as the ETP stage of T-cell differentiation, consistent with expansion of this early population of cells.
Cre is expressed and Bax is deleted in early thymocyte progenitor populations. (A) Immunofluorescence of thymocyte cells from LckCre-BaxF/+Bak−/− (control) and LckCre+BaxBakDKO mice. (B) Real-time PCR data confirming the expression of Cre in DN T-cell populations sorted as described in “Methods.” (C-D) Relative expression of intracellular Bax in DN1c-kithigh cells of control and LckCre+BaxBakDKO mice as measured by flow cytometric analysis. (E-F) Relative expression of intracellular Bax in DN3 cells of control and LckCre+BaxBak DKO mice by flow cytometric analysis.
Cre is expressed and Bax is deleted in early thymocyte progenitor populations. (A) Immunofluorescence of thymocyte cells from LckCre-BaxF/+Bak−/− (control) and LckCre+BaxBakDKO mice. (B) Real-time PCR data confirming the expression of Cre in DN T-cell populations sorted as described in “Methods.” (C-D) Relative expression of intracellular Bax in DN1c-kithigh cells of control and LckCre+BaxBakDKO mice as measured by flow cytometric analysis. (E-F) Relative expression of intracellular Bax in DN3 cells of control and LckCre+BaxBak DKO mice by flow cytometric analysis.
T-cell populations do not progress past the DN2 stage in vitro in the absence of Bax and Bak
To further establish the differentiative capacity of cells doubly deficient in Bax and Bak, we used an in vitro T-cell differentiation system established by Zuniga-Pflucker in which BM progenitor cells are cultured on OP9 stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1). In this system, BM-derived hematopoietic progenitor cells are able to commit to the T-cell lineage, undergo stage specific proliferation and mature into CD4+ and CD8+ T cells in vitro.14 This provides a system with which to examine survival and developmental outcomes of pre-T cells in the presence or absence of productive Notch signal mediated by interaction with its ligand, Delta-like 1. To determine whether BM progenitor cells lacking Bax and Bak cultured on OP9-DL1 cells could be directed to differentiate to T lymphocytes, we placed undifferentiated lineage-depleted (Gr-1–, Mac-1–, Ter119-, and B220-) BM cells from both Mx-Cre negative (control) and Mx-Cre+BaxBak DKO mice on both OP9-control and OP9-DL1 cells. Mice expressing Mx-Cre were used in these experiments to ensure that Cre would be expressed and Bax deleted in the BM progenitor population.
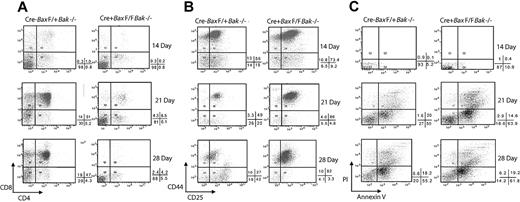
Cre− control but not Cre+ BM progenitor cells cultured on OP9-DL1 cells demonstrate CD4+CD8+ (DP) cells by day 14, and within 21 days, at least 50% of the culture is composed of DP T cells (Figure 4A). Strikingly, cultures of the progenitor cells from MxCre+BaxBak DKO mice do not differentiate to the DP stage, remaining at DN stages (Figure 4A) even up to 21 days in culture. The DN T-cell progenitors from both Cre− and Cre+ mouse cells express of CD44 and CD25 on day 14. By day 21, the control BM progenitor cultures display a normal distribution of all DN populations. Remarkably, the MxCre+BaxBak DKO culures demonstrate a dominant population of cells displaying surface markers consistent with a DN2 population (CD4CD8−CD25+CD44+; Figure 4B). These results are inconsistent with inhibition of differentiation due to depletion of growth factors by competition from mature cells that fail to die, as these cultures contain an excess of cytokines and are lacking in any appreciable number of DP cells. Interestingly, the absolute doubling time of the BaxBak DKO cultures is reduced relative to control cultures, consistent with the decreased proliferative capacity of BaxBak DKO cells previously reported for mature T cells.9 In addition, BaxBak DKO cells do not demonstrate increased apoptosis (Figure 4C) on day 14 of culture. Taken together with the flow cytometry analysis, defects observed in OP9-DL1 cultures are most consistent with a defect in early T-cell differentiation, at the ETP stage, in the absence of Bax and Bak.
BaxBak doubly deficient T-cell progenitors fail to differentiate in an OP9-DL1 culture system. Mx-Cre+BaxBak DKO and MxCre-Bax F/+Bak−/− (control) mice were treated with Poly (I; C) to induce Cre expression. Hematopoietic progenitor cells were isolated from BM and induced to differentiate over a period of 4 weeks by culture on OP9-DL1cells in the presence of interleukin-7 and fms-like tyrosine kinase receptor-3. (A) Nonadherant hematopoietic cells were collected on 14, 21, and 28 days and CD4 and CD8 expression in thymocyte progenitors were analyzed by flow cytometry. (B) CD4-CD8-Mx-Cre+BaxBak DKO and control cells were analyzed for CD25 and CD44 expression. (C) Hematopoietic progenitor cells collected from the OP9-DL1 cultures were stained with annexin V to identify and quantify apoptotic cells by flow cytometry. Percentages of annexin V and propidium iodide positive and negative quadrants are shown below each dot plot. Data are representative of 3 independent experiments.
BaxBak doubly deficient T-cell progenitors fail to differentiate in an OP9-DL1 culture system. Mx-Cre+BaxBak DKO and MxCre-Bax F/+Bak−/− (control) mice were treated with Poly (I; C) to induce Cre expression. Hematopoietic progenitor cells were isolated from BM and induced to differentiate over a period of 4 weeks by culture on OP9-DL1cells in the presence of interleukin-7 and fms-like tyrosine kinase receptor-3. (A) Nonadherant hematopoietic cells were collected on 14, 21, and 28 days and CD4 and CD8 expression in thymocyte progenitors were analyzed by flow cytometry. (B) CD4-CD8-Mx-Cre+BaxBak DKO and control cells were analyzed for CD25 and CD44 expression. (C) Hematopoietic progenitor cells collected from the OP9-DL1 cultures were stained with annexin V to identify and quantify apoptotic cells by flow cytometry. Percentages of annexin V and propidium iodide positive and negative quadrants are shown below each dot plot. Data are representative of 3 independent experiments.
To further evaluate apoptosis in the DN1cKithigh population in vivo, we evaluated annexin V+ staining in this population in LckCre+BaxBak DKO thymocytes and control thymocytes (supplemental Figure 1E). We find no change in apoptosis in DN1cKithigh thymocytes upon deletion of Bax and Bak. These results indicate that the accumulation of DN1ckithigh cells in LckCre+BaxBak DKO mice is not due to a decrease in apoptosis of these cells.
Development of T-cell lymphoma in BaxBak-deficient mice
The early lethality of BaxBak DKO mice precludes assessment of tumorigenesis in the absence of Bax and Bak. In the setting of a T-cell specific deletion of Bax and Bak, mice develop an aggressive T-cell malignancy closely resembling human T-ALL) with a median survival of 10.0 months (Figure 5A). Immunophenotype analysis demonstrates that the tumor cells are CD4−CD8−CD44+ and CD4 SP cells (Figure 5C). To determine whether these tumors arise from cells that are pre- or post-β chain selection, we isolated genomic DNA from tumors and performed PCR to determine the Dβ2 to Jβ2 rearrangements. BaxBak DKO tumors display a PCR product consistent with beta chain rearrangement (Figure 5B). The absence of a dominant PCR product suggests that these tumors are oligoclonal. Peripheral blood and BM demonstrate accumulation of malignant lymphoblasts (Figure 5D). Bax and Bak are therefore required to prevent tumorigenesis in the T-cell compartment.
Survival curve and disease phenotypes of LckCre+BaxBak DKO mice develop T-ALL. (A) Kaplan-Meier survival curves for cohorts of LckCre+BaxBak DKO and control mice. The median survival of LckCre+BaxBak DKO mice was 10 months. n = 21 for both cohorts of mice, and P = .0001. (B) PCR of genomic DNA of LckCre+BaxBak DKO tumors for rearrangements at the TCRβ locus using Dβ2 and Jβ2 region-specific primers. LckCre+BaxBak DKO tumors display clonal beta chain rearrangement. (C) Representative immunophenotype of T-ALL arising in LckCre+BaxBak DKO mice. (D) Blood smear and cytospin of BM from a representative LckCre+BaxBak DKO tumor.
Survival curve and disease phenotypes of LckCre+BaxBak DKO mice develop T-ALL. (A) Kaplan-Meier survival curves for cohorts of LckCre+BaxBak DKO and control mice. The median survival of LckCre+BaxBak DKO mice was 10 months. n = 21 for both cohorts of mice, and P = .0001. (B) PCR of genomic DNA of LckCre+BaxBak DKO tumors for rearrangements at the TCRβ locus using Dβ2 and Jβ2 region-specific primers. LckCre+BaxBak DKO tumors display clonal beta chain rearrangement. (C) Representative immunophenotype of T-ALL arising in LckCre+BaxBak DKO mice. (D) Blood smear and cytospin of BM from a representative LckCre+BaxBak DKO tumor.
LckCre+BaxBak DKO thymocytes are resistant to apoptosis
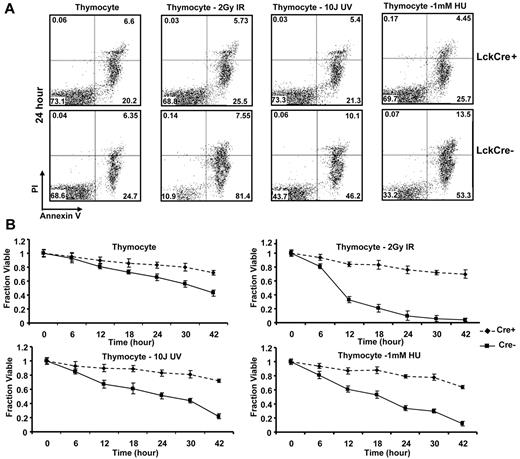
One of the hallmarks of cancer is the development of a defect in apoptosis. Multidomain proapoptotic Bax and Bak are essential effectors responsible for the permeabilization of the mitochondrial outer membrane during apoptosis. Upon initiation of a death stimulus, the BH3-only Bcl-2 family members, including truncated Bid (tBid), Bim, and Puma, activate Bax and Bak to mediate cytochrome c efflux, leading to caspase activation.17 Cell death in thymocyte development is initiated through both TCR stimulation as well as DNA damage arising through replicative stress or production of DNA strand breaks during TCR rearrangement. In the absence of Bax and Bak, T cells are profoundly defective in cell death after antigen stimulation. We therefore evaluated the effect of apoptotic stimuli inducing DNA damage through strand breaks (IR or UV irradiation), or replicative stress (HU) on isolated thymocytes from LckCre+BaxBakDKO mice. In culture, thymocytes from LckCre+BaxBak DKO mice demonstrated decreased annexin V positive cells (Figure 6A-B). After treatment with IR, HU, and UV, control thymocytes but not LckCre+BaxBak DKO thymocytes demonstrated increased annexin V possitivity, consistent with a profound defect in apoptosis in the absence of Bax and Bak (Figure 6B).
Thymocyte cells from LckCre+BaxBak DKO mice are resistant to DNA damage-induced apoptotic signals. Thymocytes from LckCre+BaxBak DKO and control mice were treated with 1mM HU, 2 Gy IR, and 10 J UV as indicated. (A) Cell viability was determined by annexin V staining with propidium iodine exclusion followed by flow cytometry at the indicated time points. Representative flow cytometric plots are shown at the 24-hour time point. (B) Quantification of the average cell survival (annexin V negative) from duplicate samples by flow cytometric analysis at the indicated time points. Data are representative of at least 2 independent experiments.
Thymocyte cells from LckCre+BaxBak DKO mice are resistant to DNA damage-induced apoptotic signals. Thymocytes from LckCre+BaxBak DKO and control mice were treated with 1mM HU, 2 Gy IR, and 10 J UV as indicated. (A) Cell viability was determined by annexin V staining with propidium iodine exclusion followed by flow cytometry at the indicated time points. Representative flow cytometric plots are shown at the 24-hour time point. (B) Quantification of the average cell survival (annexin V negative) from duplicate samples by flow cytometric analysis at the indicated time points. Data are representative of at least 2 independent experiments.
LckCre+BaxBak DKO leukemic cells are resistant to cell death
The 10-month latency of BaxBak DKO tumors suggests that additional mutations have occurred during transformation to malignancy. To investigate whether these additional mutations may cooperate with BaxBak-deficiency to allow sensitivity to cell death, we tested the sensitivity of BaxBak DKO tumor cells to DNA damage-induced cell death. BaxBak DKO tumor cells and control thymocytes were treated with IR, etoposide, HU, and UV as per Figure 6. BaxBak DKO tumor cells are resistant to cell death induced by IR, etoposide, HU, and UV (supplemental Figure 2), indicating that BaxBak DKO tumors have not acquired sensitivity to DNA damage-induced cell death.
LckCre+BaxBak DKO tumors do not demonstrate activation of the Notch pathway
The profound defect in apoptosis of BaxBak DKO cells may facilitate progression to malignancy by persistence of thymocytes with potential for the accumulation of additional mutations. BaxBak-deficient cells display defects in the ability to proliferate,9 suggesting that BaxBak-deficient leukemic cells have acquired an increased proliferative signal that drives the aggressive T-cell malignancy in these mice. One of the major signaling pathways up-regulated in human T-cell ALL is the Notch pathway. To determine whether BaxBak-deficient tumors rely on signaling through the Notch pathway, we evaluated tumors for evidence of amplification of key members of the Notch pathway by reverse transcription PCR. Interestingly, the majority of the samples demonstrated no evidence of increased expression of Notch1 (Figure 7), or the Notch targets hairy-enhancer of split 1, or Deltex1, a mammalian homolog of Drosophila Deltex.18 Of note, several mice developed tumors that were found to have normal expression of Bax, likely due to incomplete excision by Lck-Cre, and these tumors expressed high levels of Notch1. Our data are consistent with a role for Bax and Bak in preventing T-cell leukemogenesis. Furthermore, tumors arising in the absence of Bax and Bak do not require activation of the Notch pathway.
LckCre+BaxBak DKO tumors do not demonstrate activation of the Notch pathway. Total RNA was isolated from tumor and healthy T cells from LckCre+BaxBak DKO and wild type mice. Real-time PCR analysis was performed and expression levels of the Notch1 signaling pathways genes; Notch1, Deltex1, hairy-enhancer of split 1 were normalized with actin and compared. LckCre+BaxBak DKO tumors that demonstrate efficient excision of Bax do not demonstrate evidence of activated Notch signaling. The horizontal bars indicate the medium value, and the black points (■ healthy WT; ▴ healthy DKO; ▾ tumor Wt;♦ tumor DKO) delineate the overall range of expression of the indicated genes. **P < .05, and ***P < .01.
LckCre+BaxBak DKO tumors do not demonstrate activation of the Notch pathway. Total RNA was isolated from tumor and healthy T cells from LckCre+BaxBak DKO and wild type mice. Real-time PCR analysis was performed and expression levels of the Notch1 signaling pathways genes; Notch1, Deltex1, hairy-enhancer of split 1 were normalized with actin and compared. LckCre+BaxBak DKO tumors that demonstrate efficient excision of Bax do not demonstrate evidence of activated Notch signaling. The horizontal bars indicate the medium value, and the black points (■ healthy WT; ▴ healthy DKO; ▾ tumor Wt;♦ tumor DKO) delineate the overall range of expression of the indicated genes. **P < .05, and ***P < .01.
Discussion
Although the critical role of the intrinsic pathway of apoptosis in T-cell development during positive and negative selection is well established, the role of apoptosis in early T-cell development and lineage commitment is less well understood. We have demonstrated a critical role for proapoptotic Bax and Bak remarkably early in T-cell development, before beta chain selection, manifest by altered thymocyte populations as well as a failure of BaxBak-deficient BM progenitor cells to differentiate in OP9-DL1 cultures. Interestingly, BM progenitor cells from mice in which Bax and Bak have been deleted in the germ line are capable of producing DP cells when transplanted into Rag−/− mice, but are unable to maintain normal T-cell differentiation over time.8 This may reflect the presence of factors in an in vivo environment that can compensate at least initially for the BaxBak-mediated role in differentiation. Alternatively, deletion of Bax and Bak in the germ line may select for compensatory signals that are not present when Bax and Bak are deleted in adult mice. Our system clearly demonstrates a role for Bax and Bak in early T-cell development, and should provide a basis for additional experiments to clarify the mechanism by which these proteins influence early T-cell development.
The increased DN thymocyte population in BaxBak-deficient mice may be due to failure of apoptosis to control homeostasis of this early T-cell compartment. Alternatively, these cells may represent DP T cells that fail to undergo negative selection due to the severe defect in apoptosis in the absence of Bax and Bak, and subsequently down-regulate their TCR. We note the presence of some Beta chain rearrangement in this DN population consistent with a post-beta chain rearrangement stage origin. Nonetheless, hematopoietic progenitor cells that are deficient for Bax and Bak fail to differentiate in in vitro OP9-DL1 cultures, consistent with an additional accumulation of early T-cell progenitors due to an inability of these cells to undergo proper differentiation. We thus establish a critical role for Bax and Bak in early T-cell differentiation.
Interestingly, BaxBak-deficient mice develop an aggressive T-cell malignancy that does not demonstrate amplification of the Notch pathway. These results suggest that the absence of Bax and Bak promotes transformation of T cells in a manner distinct from amplification of Notch signaling. Further studies to determine progenitor cell that is transformed in these mice may provide important insights into the mechanism by which loss of Bax and Bak promotes transformation.
How might Bax and Bak impact early T-cell differentiation? Activation of Bax and Bak results in the release of cytochrome c, culminating in cell death. However, cells must survive to differentiate. Accumulating evidence suggests that caspases may regulate cellular functions distinct from cell death, including T-cell activation, innate immunity, and HSC function.19,20 It is therefore possible that Bax and Bak also serve to activate these nonapoptotic functions of caspases, thereby impacting differentiation. Alternatively, Bax and Bak may play a role at the mitochondria distinct from the release of cytochrome c required for proper T-cell development. Further studies will be necessary to dissect the role of Bax and Bak in this setting.
In conclusion, we have demonstrated a critical role for Bax and Bak in T-cell differentiation. In the absence of Bax and Bak, thymocytes display abnormal differentiation and BM progenitors fail to differentiate in OP9-DL1 cultures. These studies demonstrate a novel role for proapoptotic Bax and Bak remarkably early in T-cell differentiation, to promote proper differentiation of early T-cell progenitors and prevent transformation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Jennifer Pietenpol, Scott Hiebert, Mark Boothby, Keunwook Lee, and Elizabeth Yang for helpful discussions, the late Stanley J. Korsmeyer for the conditional BaxBak double knockout mouse, J.C. Zuñiga-Pflücker (University of Toronto) for the OP9-DL1 cell line, and Kim Johnson for help with thymocyte immunofluorescence. We thank Drs Kevin P. Weller and David Flaherty for flow cytometry assistance.
This work was supported by the Martell Foundation, G&P Scholar Award, and Kimmel Scholar Award to S.S.Z.
National Institutes of Health
Authorship
Contributions: S.B. designed and performed research, analyzed data, and co-wrote the manuscript; Q.S., L.M., and S.C. performed research, analyzed data, and provided input on the manuscript; U.D. supervised S.C., analyzed data, and provided input on the manuscript; and S.Z. supervised experiments, designed research, analyzed data, and co-wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandra Zinkel, Department of Medicine, Vanderbilt University Medical Center, 2220 Pierce Ave, PRB 548, Nashville, TN 37232; e-mail: sandra.zinkel@vanderbilt.edu.