Abstract
Two critical concerns in clinical cord blood transplantation are the initial time to engraftment and the subsequent restoration of immune function. These studies measured the impact of progenitor cell dose on both the pace and strength of hematopoietic reconstitution by transplanting nonobese diabetic/severe combined immunodeficiency/interleukin-2 receptor-gamma–null (NSγ) mice with lineage-depleted aldehyde dehydrogenase-bright CD34+ human cord blood progenitors. The progress of each transplant was monitored over an extended time course by repeatedly analyzing the peripheral blood for human hematopoietic cells. In vivo human hematopoietic development was complete. After long-term transplantation assays (≥ 19 weeks), human T-cell development was documented within multiple tissues in 16 of 32 NSγ mice. Human T-cell differentiation was active within NSγ thymuses, as documented by the presence of CD4+ CD8+ T-cell progenitors as well as T-cell receptor excision circles. It is important to note that although myeloid and B-cell engraftment was detected as early as 4 weeks after transplantation, human T-cell development was exclusively late onset. High progenitor cell doses were associated with a robust human hematopoietic chimerism that accelerated both initial time to engraftment and subsequent T-cell development. At lower progenitor cell doses, the chimerism was weak and the human hematopoietic lineage development was frequently incomplete.
Introduction
Umbilical cord blood transplantation (CBT) provides a successful alternative therapeutic option for transplant patients who have no suitable related allogeneic donor. However, one profound complication to CBT has been the widely shared experience of significant delays in the recovery of all hematopoietic lineages.1-8 In patients who receive CBT, the initial time-to-myeloid engraftment is typically 1 month. The development of donor-derived B and T cells is most commonly not observed until 6 months after transplant or longer. Factors that accelerate the rate of hematopoietic engraftment and immune reconstitution would increase the effectiveness and safety of CBT.
There are clear indications that effective CBT relies on the content of progenitors within individual CB grafts. The total dose of CD34+ cells within a CB unit has been associated with patient survival.9 Moreover, the total dose of clonogenic progenitors within the graft correlates with engraftment.6,7,10 Furthermore, data from experimental murine models for bone marrow transplantation suggest that the pace and strength of hematopoietic reconstitution rely on long-term repopulating cells as well as on other “short-term” progenitors that make rapid contributions to hematopoietic development.11-20
The intent of this study was to evaluate the impact that the dose of transplantable CB progenitors exerts on the pace and strength of hematopoietic engraftment. These studies capitalized on methods that we previously developed to purify hematopoietic progenitors, based on their high expression of aldehyde dehydrogenase (ALDHbr).21-23 The ALDHbr cell fraction captures essentially the entire content of both short- and long-term nonobese diabetic/severe combined immunodeficiency (NOD/SCID)-repopulating cells (SRCs) from CB.23-26 Therefore, the ALDHbr cell fraction contains a mixture of progenitors with potential clinical significance.
In the current studies, xenograft transplantation assays were performed using NOD/SCID–interleukin-2 receptor-γ–null (IL-2R-γnull; NSγ) mice.27-33 For the purpose of evaluating clinical strategies for CBT, the NSγ xenograft model offers several key advantages. First, human hematopoietic chimerism in NSγ mice is robust and can be evaluated in the peripheral blood. This makes it possible to monitor the progress of each transplant over time, in much the same way that clinical transplants are evaluated. Furthermore, NSγ mice support a broad range of human hematopoietic development, including human T-cell development,27,28,34 whereas studies that use other NOD/SCID strains evaluate human lymphoid engraftment based solely on B cells.23,35-38
By taking repeated, serial measurements from the blood of each mouse that was transplanted, a relationship was established between the progenitor cell dose and the pace of human hematopoietic engraftment. Lineage depleted (Linneg) ALDHbr CD34+ cells efficiently developed into T cells, which were detected within the NSγ bone marrow, spleen, thymus, and peripheral blood. Importantly, human T-cell development was not observed within the first 12 weeks after transplantation and provided a means to evaluate late-onset lymphocyte development. In total, these studies indicated that both the level and pace of hematopoietic engraftment and immune recovery were dependent upon the dose of purified progenitors that were administered in the transplant.
Methods
Fluorescent reagents
Antibody reagents to detect human CD3 (clone SK7), CD8 (clone SK1), and CD33 (clone P67.6) were purchased from BD Biosciences. All other antibody reagents were purchased from BD Pharmingen, including antibodies specific for human CD4 (clone SK3), CD14 (clone M5E2), CD19 (clone HIB19), CD34 (clone 581), CD45 (clone HI30) CD56 (clone B159), and murine CD45 (clone 30-F11).
Cell purification
Umbilical cord blood was obtained from the Carolinas Cord Blood Bank, under protocols approved by the Duke Institutional Review Board. Mononuclear cells were enriched by density separation on Ficoll-Hypaque (Lymphoprep; Axis-Shield), and lineage-committed CB cells were removed using immunomagnetic separation (StemSep progenitor enrichment; StemCell Technologies). As previously described, Linneg CB cells at 106 cells/mL were stained with 1μM Aldefluor (StemCell Technologies) in Dulbecco phosphate-buffered saline supplemented with 1% fetal calf serum (FCS), 500μM EDTA (ethylenediaminetetraacetic acid), and 50μM verapamil (staining buffer).23 After 30 minutes at 37°C, the cells were pelleted by centrifugation, placed on ice, and stained with fluorescence-conjugated antibodies against CD34 for 15 minutes. The cells were then washed with chilled staining buffer and held on ice throughout fluorescence-activated cell sorting (FACS). Linneg ALDHbr CD34+ cells were purified using a FACSVantage Cell Sorter (BD Biosciences). To rigorously purify the progenitors, all cell fractions were immediately resorted.
Cell transplantation assays
All cell transplantation assays were performed per protocols approved by the Institutional Animal Care and Use Committee at Duke University. NSγ mice were purchased from Jackson Laboratories.28 Then, 2-6 hours before transplantation, the mice received sublethal irradiation from a 137Cs source (325 cGy). The purified cell grafts were prepared in Iscove modified Dulbecco medium supplemented with 2% FCS and were transplanted by tail-vein injection.
Posttransplantation analyses
Beginning at 4 weeks after transplantation, peripheral blood (50 μL) from transplanted mice was sampled at 3-week intervals to analyze its relative content of human cells. Each blood sample was used in a single staining reaction. The human CD45+ white cells were simultaneously analyzed for their relative expression of human CD3, CD8, CD19, and CD56. Murine CD45+ cells were excluded from each analysis.
Fluorescent beads were added to each sample (50 μL; Flow-Count Fluorospheres; Beckman-Coulter). Calculations for the absolute counts for human CD45+ cells within the mouse peripheral blood cells used the following formula: absolute huCD45+ cells/μL blood = [(total number of huCD45+ cells detected)/(total number of beads detected)]*(beads/μL)
After 19-21 weeks, the mice were killed to analyze the bone marrow, spleen, and thymus for their total content of human CD45+ hematopoietic cells. In the bone marrow and spleen, the human CD45+ hematopoietic cells were characterized for their expression of CD3 (T cells), CD19 (B cells), CD56 (natural killer [NK] cells), CD34 (progenitors), and CD33, CD13, and/or CD14 (myeloid cells). In the NSγ thymus, human CD45+ CD3+ T cells were analyzed for their relative expression of CD4 and CD8. Murine CD45+ cells were excluded from each analysis.
Retransplantation assays
In some studies, human CD34+ progenitors were enriched from the bone marrow of primary NSγ transplant recipients to transplant them into secondary NSγ mice. Single-cell suspensions were prepared from bone marrow, and the mature murine cells were removed by immunomagnetic lineage depletion (EasySep murine progenitor enrichment; StemCell Technologies). Mature human cells were then depleted by immunomagnetic lineage depletion (EasySep human progenitor enrichment; StemCell Technologies).
T-cell proliferation assays
Human CD3+ T cells were isolated from NSγ spleens by FACS and were cultured in RPMI 1640 medium supplemented with 10% FCS, 5.5μM 2-mercaptoethanol, IL-2 (5 ng/mL), and IL-7 (20 ng/mL) (R&D Systems). The T cells were stimulated with antibodies specific for CD3 and CD28 that were immobilized on beads (Dynabeads; Invitrogen) in cultures established at a ratio of 4 cells/bead, with or without irradiated human umbilical vein endothelial cells (HUVECs) cells (20 Gy; Invitrogen). After 7 days, the cultures were analyzed by flow cytometry. To quantify the cell recovery from each culture, Flow-Count Fluorospheres (5 μL; Beckman-Coulter) were added to each sample. Calculations for the absolute counts for human CD3+ cells used the formula: total huCD3+ cells/culture = [(number of huCD3+ cells detected)/(number of beads detected)]*(beads/5 μL).
Statistical analyses
The pace of engraftment was measured based on the presence of human cells in the peripheral blood of NSγ mice. Quantitative and qualitative measurements were taken over an extended time course after transplantation. Comparisons drawn between different time points used 2-tailed t tests, and samples were considered matched and equivalent. Short-term SRCs were defined at 7 weeks after transplantation by the presence of human CD45+ cells within the peripheral blood. Long-term SRCs were based on demonstrating complete human hematopoietic development within the mouse bone marrow, as defined in the text. SRC frequencies were estimates of maximum likelihood within a single-hit Poisson distribution and were prepared using LCalc Version 1.1 software (StemCell Technologies), which also provides a confidence interval (95% CI).
Results
Early human hematopoietic engraftment in NSγ mice
To perform transplantation studies in NSγ mice, CB was first depleted of mature cells to enrich progenitors (Figure 1). Linneg ALDHbr CD34+ cells were then purified by FACS. To ensure purity, each Linneg ALDHbr CD34+ cell preparation was immediately repurified. Sublethally irradiated (325 cGy) NSγ mice were then transplanted with 300-30 000 Linneg ALDHbr CD34+ cells. The progress of each individual transplant was monitored by the emergence of human CD45+ cells in the peripheral blood (Figure 1). The blood (50 μL) was analyzed, beginning at 4 weeks after transplantation, and was reanalyzed at 3-week intervals across an extended time course (19-21 weeks). Perhaps because of the nature of NSγ mice, the human cells detected within the blood were predominantly lymphocytes.
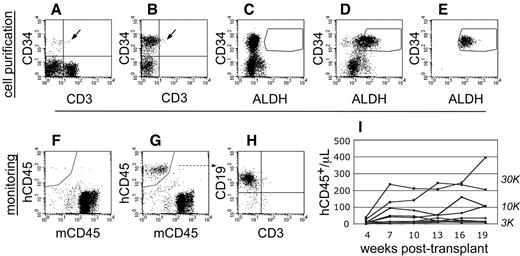
Cell purification and transplantation of NSγ mice. (A-E) NSγ mice were transplanted with ALDHbr CD34+ cells prepared from CB that had been depleted of mature, lineage-marked cells (Linneg CB). Total CB has a low content of CD34+ progenitors (A), and these progenitors are enriched in Linneg CB (B). Enzyme-inhibited Linneg cells (treated with diethyl aminobenzaldehyde) were used to establish baseline fluorescence for ALDH and to establish sorting gates to purify ALDHbr CD34+ cells (C). ALDHbr CD34+ cells were purified from lineage-depleted CB (D), and each cell preparation was purified twice before cell transplantation (E). (F-I) After transplantation, the human hematopoietic chimerism was monitored based on the number of human CD45+ cells detected per microliter of peripheral blood. Without human hematopoietic engraftment, the peripheral blood contained only murine CD45+ cells (F). With engraftment, human CD45+ cells are present within the peripheral blood (G). In short-term studies (≤ 10 weeks), the human CD45+ cells within the blood are predominantly CD19+ (H). Using these methods, a history of human hematopoietic engraftment could be established for each transplantation assay (I). One cohort of mice that were transplanted with progenitors derived from a single CB that were transplanted into multiple different mice at multiple different cell doses.
Cell purification and transplantation of NSγ mice. (A-E) NSγ mice were transplanted with ALDHbr CD34+ cells prepared from CB that had been depleted of mature, lineage-marked cells (Linneg CB). Total CB has a low content of CD34+ progenitors (A), and these progenitors are enriched in Linneg CB (B). Enzyme-inhibited Linneg cells (treated with diethyl aminobenzaldehyde) were used to establish baseline fluorescence for ALDH and to establish sorting gates to purify ALDHbr CD34+ cells (C). ALDHbr CD34+ cells were purified from lineage-depleted CB (D), and each cell preparation was purified twice before cell transplantation (E). (F-I) After transplantation, the human hematopoietic chimerism was monitored based on the number of human CD45+ cells detected per microliter of peripheral blood. Without human hematopoietic engraftment, the peripheral blood contained only murine CD45+ cells (F). With engraftment, human CD45+ cells are present within the peripheral blood (G). In short-term studies (≤ 10 weeks), the human CD45+ cells within the blood are predominantly CD19+ (H). Using these methods, a history of human hematopoietic engraftment could be established for each transplantation assay (I). One cohort of mice that were transplanted with progenitors derived from a single CB that were transplanted into multiple different mice at multiple different cell doses.
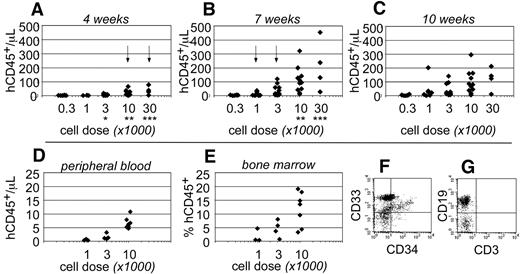
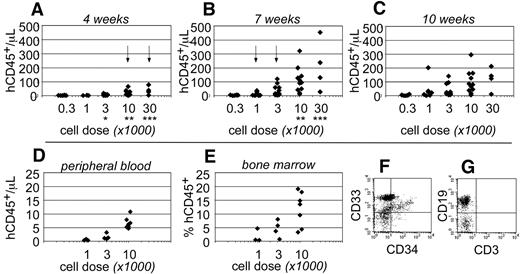
Human CD45+ hematopoietic cells were evident within the peripheral blood as early as 4 weeks after transplantation. At that time, human cells were only detected in the blood of mice that had been transplanted at the highest cell doses (≥ 10 000 cells; Figure 2A). By 7 weeks after transplantation, the total numbers of human CD45+ cells increased within the peripheral blood and could be detected at all transplant cell doses (Figure 2B). However, at the lowest cell dose (300 cells), the chimerism was near the limit of our ability to detect human cells (3 cells/μL blood). At 10 weeks after transplantation, no significant differences were observed in the total numbers of human cells that were detected within the peripheral blood (Figure 2C). These data indicated that short-term engraftment was established by 7 weeks after transplantation. Based on the human hematopoietic chimerism within the peripheral blood at that time, the frequency for short-term SRCs was 1 in every 555 Linneg ALDHbr CD34+ cells (95% CI: 1/286-1/1076).
Short-term hematopoietic engraftment in NSγ mice. (A-C) Human hematopoietic engraftment was quantified based on the number of CD45+ cells detected per microliter of peripheral blood. Three panels present these data from 4 (A), 7 (B), and 10 weeks (C) after transplantation, respectively. At each time point, the data were stratified based on the transplant doses of ALDHbr CD34+ cells that each mouse received. Arrows indicate the first time point when CD45+ cells were observed for each cell dose. *Indicates a significant difference (P < .005) in chimerism between transplant doses of 3000 and 10 000 at 4 weeks after transplantation; ** and *** indicate significant increases (P < .05) in chimerism between 4 and 7 weeks for doses of 10 000 and 30 000 cells, respectively. The levels of chimerism observed 10 weeks after transplantation were not significantly different from those observed 7 weeks after transplantation. (D-G) In one cohort of 14 mice, the hematopoietic chimerism to the blood was compared with engraftment to the bone marrow 4 weeks after transplantation. Human CD45+ cells were detected within the peripheral blood at the highest transplant doses (D) and in the bone marrow (E). In panels F and G, total bone marrow cells were first gated to examine human CD45+ cells that did not express murine CD45 (not shown). Those cells included CD33+ myeloid cells (F), CD34+ progenitors (F), and human CD19+ B cells (G). CD3+ T cells were not detected (G).
Short-term hematopoietic engraftment in NSγ mice. (A-C) Human hematopoietic engraftment was quantified based on the number of CD45+ cells detected per microliter of peripheral blood. Three panels present these data from 4 (A), 7 (B), and 10 weeks (C) after transplantation, respectively. At each time point, the data were stratified based on the transplant doses of ALDHbr CD34+ cells that each mouse received. Arrows indicate the first time point when CD45+ cells were observed for each cell dose. *Indicates a significant difference (P < .005) in chimerism between transplant doses of 3000 and 10 000 at 4 weeks after transplantation; ** and *** indicate significant increases (P < .05) in chimerism between 4 and 7 weeks for doses of 10 000 and 30 000 cells, respectively. The levels of chimerism observed 10 weeks after transplantation were not significantly different from those observed 7 weeks after transplantation. (D-G) In one cohort of 14 mice, the hematopoietic chimerism to the blood was compared with engraftment to the bone marrow 4 weeks after transplantation. Human CD45+ cells were detected within the peripheral blood at the highest transplant doses (D) and in the bone marrow (E). In panels F and G, total bone marrow cells were first gated to examine human CD45+ cells that did not express murine CD45 (not shown). Those cells included CD33+ myeloid cells (F), CD34+ progenitors (F), and human CD19+ B cells (G). CD3+ T cells were not detected (G).
To examine short-term engraftment in more detail, 14 mice were sacrificed 4 weeks after transplantation. Within this cohort, human hematopoietic cells were detected in the blood of mice that had been transplanted with 10 000 linneg ALDHbr CD34+ progenitors (n = 7; Figure 2D). However, with the exception of a single mouse, human cells were not observed within the blood of mice that received lower transplant doses (n = 7). In these same mice, human CD33+ myeloid cells and CD19+ B-lymphoid cells were present within the bone marrow (Figure 2F-G). Therefore, the human cells detected within the blood corresponded with multiple-lineage hematopoietic engraftment to the bone marrow. The levels of human hematopoietic engraftment to the bone marrow were proportional to the transplant cell dose (Figure 2E). Several of the mice that received low doses of progenitors exhibited a low level of hematopoietic engraftment to the bone marrow, which was not reflected within the peripheral blood. Therefore, measurements taken from the peripheral blood appeared to be a more stringent evaluation of chimerism.
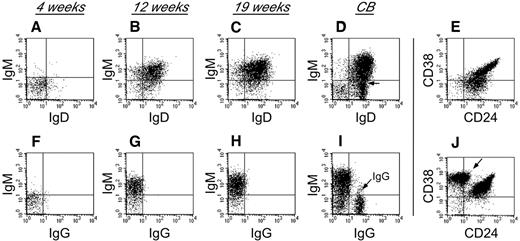
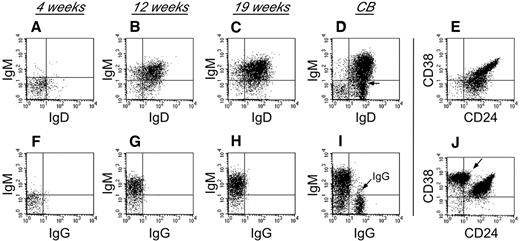
To characterize human B-cell development in NSγ mice, CD19+ cells that were present within the spleens of the transplanted NSγ mice were examined for their expression of surface immunoglobulin (Figure 3). Four weeks after transplantation, the human B cells did not exhibit any significant immunoglobulin (Ig) expression. However, by 12 weeks after transplantation, the human B cells within the spleens had developed into immature B cells that coexpressed IgM and IgD, but no surface expression of IgG was observed. The human B cells also appeared immature, based on their high coexpression of both CD24 and CD38. No further maturation was seen, even at 19 weeks after transplantation. These data suggest that human B cells do not develop completely within NSγ mice, and that the development that is supported is completed within the first 12 weeks after transplantation. Similar immature human B cells were described in the spleens of NOG (NOD/Shi-scid IL-2R-γ–null) mice that had been transplanted with 200 000 CD34+ progenitors.40 Those cells differentiated to IgG-secreting cells in vitro. In our transplants, which were performed using low doses of progenitors, the numbers of splenic IgM+ B cells that were recovered were limiting, and attempts of in vitro culture were inconclusive.
Human B-cell development in NSγ mice. Human B-cell development within spleens was characterized in NSγ mice. Four weeks after transplantation, the human CD19+ cells did not express any significant surface IgM, IgD, or IgG (panels A,F; n = 6). Twelve weeks after transplantation, the human CD19+ cells coexpressed IgM and IgD; however, surface IgG was not expressed (panels B,G; n = 5). At 19 weeks after transplantation, the human CD19+ cells also coexpressed IgM and IgD; but surface IgG was not expressed (panels C,H; n = 7). In control samples, CD19+ B cells from CB contain subpopulations that coexpress IgM and IgD (D), but also contain cells that express IgG (I, see arrow). The CD19+ human B cells within NSγ spleens coexpress intermediate to high levels of CD24 and CD38 at both 12 (not shown) and 19 weeks after transplantation (E). CD19+ CB cells contain immature CD24+ CD38+ B subsets of cells, but also contain CD38br CD24neg B cells that are presumably mature (J, see arrow). In each panel, total murine spleen cell preparations were initially gated to examine human CD45+ CD19+ cells that did not express murine CD45 (not shown). In control panels, total CB cell preparations were similarly gated to examine CD45+ CD19+ cells (not shown).
Human B-cell development in NSγ mice. Human B-cell development within spleens was characterized in NSγ mice. Four weeks after transplantation, the human CD19+ cells did not express any significant surface IgM, IgD, or IgG (panels A,F; n = 6). Twelve weeks after transplantation, the human CD19+ cells coexpressed IgM and IgD; however, surface IgG was not expressed (panels B,G; n = 5). At 19 weeks after transplantation, the human CD19+ cells also coexpressed IgM and IgD; but surface IgG was not expressed (panels C,H; n = 7). In control samples, CD19+ B cells from CB contain subpopulations that coexpress IgM and IgD (D), but also contain cells that express IgG (I, see arrow). The CD19+ human B cells within NSγ spleens coexpress intermediate to high levels of CD24 and CD38 at both 12 (not shown) and 19 weeks after transplantation (E). CD19+ CB cells contain immature CD24+ CD38+ B subsets of cells, but also contain CD38br CD24neg B cells that are presumably mature (J, see arrow). In each panel, total murine spleen cell preparations were initially gated to examine human CD45+ CD19+ cells that did not express murine CD45 (not shown). In control panels, total CB cell preparations were similarly gated to examine CD45+ CD19+ cells (not shown).
Late-onset T-cell development in NSγ mice
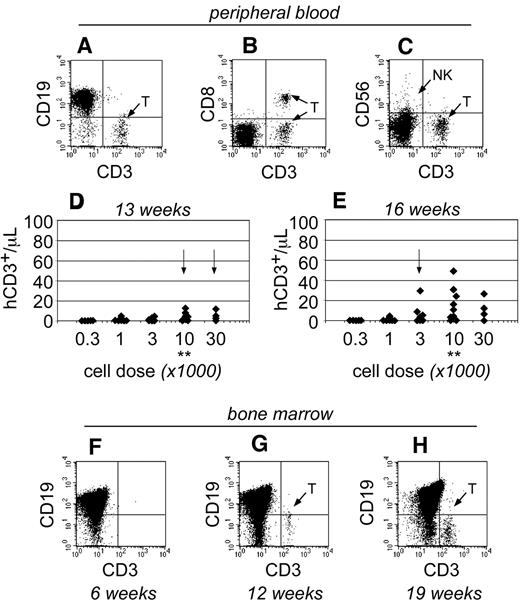
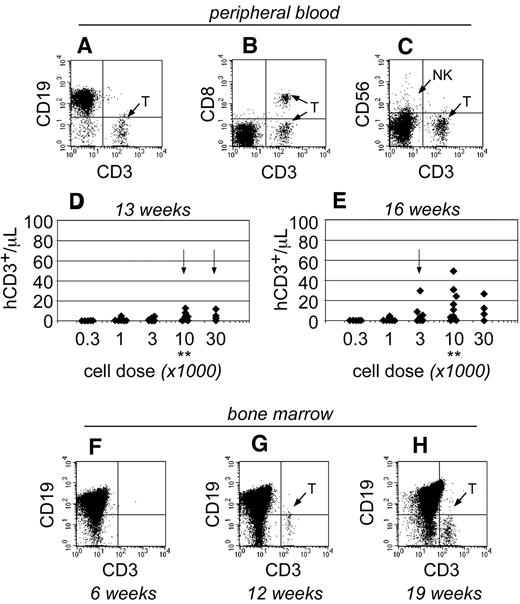
As the transplants progressed, human T cells became detectable within the peripheral blood of NSγ mice only after 13 weeks after transplantation (Figure 4). At that time, the T cells were only detectable in mice that had been transplanted with high doses of Linneg ALDHbr CD34+ cells (≥ 10 000 cells; Figure 4D). By 16 weeks after transplantation, the numbers of human T cells increased within the peripheral blood and were detectable within the blood of mice that had been transplanted with as few as 1000 Linneg ALDHbr CD34+ cells (Figure 4E). In mice that had been transplanted with 10 000 Linneg ALDHbr CD34+ progenitors, human T cells were not detectable within the bone marrow of mice by either 4 (Figure 2G) or 6 weeks after transplantation (Figure 4F). However, human CD3+ T cells were detected within the bone marrow 12 weeks after transplantation. Therefore, data obtained from both the peripheral blood and bone marrow demonstrate that human T-cell development was exclusively late onset. Importantly, the pace for the emergence of T cells was related to the transplant cell dose, just as had been observed in the studies regarding short-term hematopoietic chimerism.
The emergence of human T cells to the peripheral blood of NSγ mice. (A-E) For each mouse, the numbers of human CD45+ CD3+ T cells were quantified per microliter of peripheral blood (A), which coexpress canonical T-cell antigens, such as CD8 (B), but do not coexpress CD56, a defining antigen for NK T cells (C). With high transplant cell doses (≥ 10 000 cells), T cells began to emerge as early as 13 weeks after transplantation (D). By 16 weeks after transplantation, human T cells could be detected in the peripheral blood of mice that had been transplanted with ≥ 1000 cells (E). In panels D and E, the data were stratified based on the transplant dose of Linneg ALDHbr CD34+ cells. Arrows indicate the first time T cells are observed for each dose. **Indicates T-cell development increased significantly at a dose of 10 000 cells (P < .05). (F-H) The human CD45+ cells within the bone marrow of NSγ mice were characterized for the presence of human CD3+ T cells. T cells were not present 6 weeks after transplantation (F; n = 5), but were present at 12 (G; n = 5) and 19-21 weeks (H; n = 16 of 32) after transplantation.
The emergence of human T cells to the peripheral blood of NSγ mice. (A-E) For each mouse, the numbers of human CD45+ CD3+ T cells were quantified per microliter of peripheral blood (A), which coexpress canonical T-cell antigens, such as CD8 (B), but do not coexpress CD56, a defining antigen for NK T cells (C). With high transplant cell doses (≥ 10 000 cells), T cells began to emerge as early as 13 weeks after transplantation (D). By 16 weeks after transplantation, human T cells could be detected in the peripheral blood of mice that had been transplanted with ≥ 1000 cells (E). In panels D and E, the data were stratified based on the transplant dose of Linneg ALDHbr CD34+ cells. Arrows indicate the first time T cells are observed for each dose. **Indicates T-cell development increased significantly at a dose of 10 000 cells (P < .05). (F-H) The human CD45+ cells within the bone marrow of NSγ mice were characterized for the presence of human CD3+ T cells. T cells were not present 6 weeks after transplantation (F; n = 5), but were present at 12 (G; n = 5) and 19-21 weeks (H; n = 16 of 32) after transplantation.
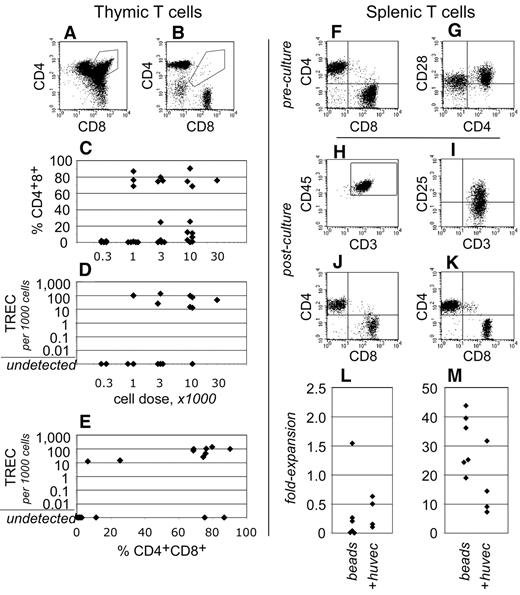
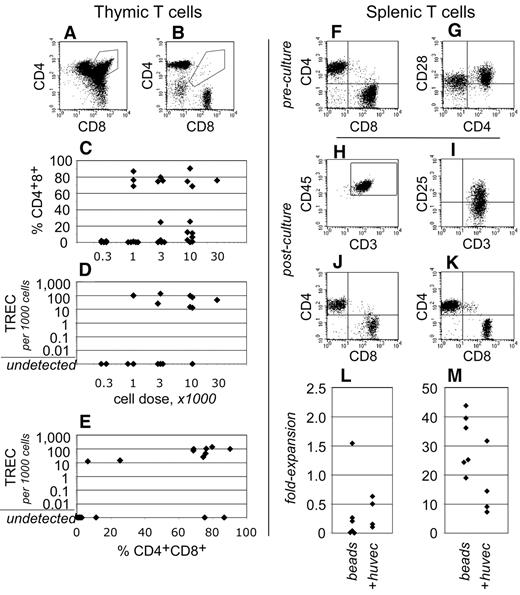
To gain insight into human T-cell development within these transplantation assays, the thymuses of NSγ mice were examined after long-term transplantation assays (19-21 weeks). Human CD3+ T cells were present within the thymus in 19 of 32 NSγ mice examined (Figure 5). In 12 of these mice, greater than 25% of the CD3+ T cells present were CD4+ CD8+ “double-positive” T-cell progenitors (Figure 5A,C). These cells represent a well-defined, transient stage of thymic T-cell development. In addition, in 9 of 18 mice that were assayed, the thymuses contained human TRECs (Figure 5D). These residual DNA episomes indicate recent T-cell receptor gene rearrangements.39 TREC episomes do not replicate, and, after T-cell receptor rearrangements are complete, they are lost gradually with each cell division. Of note, in 8 of the 9 TREC+ mice, the CD4+ CD8+ cells represented greater than 25% of the human T cells present (Figure 5E). Thus, 2 independent and transient measures for active T-cell development were detected within the same cells. These data demonstrate that T-cell development was active and derived from progenitors, rather than from the peripheral expansion of mature T cells.
Human T-cell development in NSγ mice. (A-E) The development of human T cells was characterized in NSγ thymuses. In some thymuses, the human CD45+ CD3+ T cells included CD4+ CD8+ “double-positive” T-cell progenitors (A), whereas other thymuses contained phenotypically mature “single-positive” T cells (B). High percentages of CD4+ CD8+ T-cell progenitors were observed in thymuses across a range of transplant cell doses (C). Similarly, high copy numbers of human TRECs could be detected in thymus cell preparations across a range of transplant cell doses (D). In 8 of 9 mice that expressed TRECs, at least 25% of the CD3+ cells were CD4+ CD8+ T-cell progenitors (E). (F-M) Human CD3+ T cells isolated from NSγ spleens were tested for their responsiveness to mitogens. In spleen cell preparations, the human CD3+ cells had matured into CD4+ or CD8+ “single-positive” T cells, which expressed CD28 (F-G). The T cells were costimulated using immobilized antibodies directed against CD3 and CD28 and cultured with (n = 6) or without irradiated HUVEC cells (20 Gy; n = 4). After 7 days, human CD45+ CD3+ T cells were present in the cultures, and expressed the CD25 (IL-2Rα) activation antigen (H-I). Both CD4+ and CD8+ were detected; however, most frequently, there was a net loss of T cells in the cultures (J,L). Cultures established CD3+ CB T cells contained similar CD4+ and CD8+ cells, but the numbers of cells increased dramatically in 7 days (K,M).
Human T-cell development in NSγ mice. (A-E) The development of human T cells was characterized in NSγ thymuses. In some thymuses, the human CD45+ CD3+ T cells included CD4+ CD8+ “double-positive” T-cell progenitors (A), whereas other thymuses contained phenotypically mature “single-positive” T cells (B). High percentages of CD4+ CD8+ T-cell progenitors were observed in thymuses across a range of transplant cell doses (C). Similarly, high copy numbers of human TRECs could be detected in thymus cell preparations across a range of transplant cell doses (D). In 8 of 9 mice that expressed TRECs, at least 25% of the CD3+ cells were CD4+ CD8+ T-cell progenitors (E). (F-M) Human CD3+ T cells isolated from NSγ spleens were tested for their responsiveness to mitogens. In spleen cell preparations, the human CD3+ cells had matured into CD4+ or CD8+ “single-positive” T cells, which expressed CD28 (F-G). The T cells were costimulated using immobilized antibodies directed against CD3 and CD28 and cultured with (n = 6) or without irradiated HUVEC cells (20 Gy; n = 4). After 7 days, human CD45+ CD3+ T cells were present in the cultures, and expressed the CD25 (IL-2Rα) activation antigen (H-I). Both CD4+ and CD8+ were detected; however, most frequently, there was a net loss of T cells in the cultures (J,L). Cultures established CD3+ CB T cells contained similar CD4+ and CD8+ cells, but the numbers of cells increased dramatically in 7 days (K,M).
In contrast to what had been observed in NSγ thymuses, the human T cells that were present within the NSγ spleens had differentiated into either CD4+ or CD8+ “single-positive” T cells (Figure 5F). In addition, human TRECs were not detected within preparations of 500 000 total NSγ spleen cells (n = 11). These data indicate that, at least in long-term transplantation assays, the T cells that had recently emigrated from the thymus to the spleen represented an undetectable proportion of the total splenic T cells.
Human CD3+ cells within NSγ spleens expressed CD28 (Figure 5G); therefore, immobilized antibodies specific for CD3 and CD28 were used to test whether these cells respond to mitogenic signals (n = 6). Based on reports that endothelial cells enhance T-cell stimulation, some cultures were established on irradiated monolayers of HUVECs (n = 4).41 As had been experienced with IgM+ B cells, the numbers of T cells that could be harvested from any spleen were limiting because of the low transplant doses. Proliferation cultures were established using between 2000 and 12 000 cells. Only a limited number of T cells formed the characteristic cell clusters that indicate T-cell stimulation by these beads. Most of the cells did not respond (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). After 7 days, the stimulated T cells were harvested and characterized by flow cytometry. Fluorescent microbeads were used to quantify the total numbers of cells within each culture. Although human T cells could be detected after 7 days in culture, fewer T cells were recovered than had been used to establish the cultures (Figure 5H-J,L). Irradiated HUVECs did not increase the stimulation that was observed. By comparison, the majority of CB T cells responded to stimulation (supplemental Figure 1) and proliferated between 20- and 40-fold (Figure 5K,M).
Complete multiple-lineage engraftment to NSγ bone marrow
At 19-21 weeks after transplantation, human hematopoietic development was evident within the bone marrow of 27 of 32 NSγ mice (Figure 6A). The levels of chimerism that were observed were dependent upon the transplant cell doses. It was particularly significant that 16 mice exhibited high levels of human hematopoietic chimerism within the bone marrow that included human T cells (Figure 6B-E). In these mice, the multiple-lineage chimerism within the bone marrow included human CD13+ myeloid cells, CD19+ B cells, and CD34+ progenitors (Figure 6C,F). Human NK cells were usually present at low frequencies within the bone marrow and spleen (Figure 6E), although NK cells were occasionally undetectable.
Complete hematopoietic development in NSγ mice. After long-term transplantation assays (≥ 19 weeks), human hematopoietic engraftment was analyzed in 32 NSγ mice. In total, 27 mice had detectable human hematopoietic cells, and the levels of chimerism were dose dependent (A). Complete hematopoietic development was documented in 16 NSγ mice, as defined by human T-cell development in multiple tissues. These mice exhibited high levels of chimerism and were predominately mice that had been transplanted with high doses of Linneg ALDHbr CD34+ cells (B). The human CD45+ cells within the bone marrow of these mice included CD19+ B cells (C), CD3+ T cells (C-E), CD56+ NK cells (E), CD13+ myeloid cells, and CD34+ progenitors (F). The CD3+ cells coexpressed canonical T-cell antigens (CD8; D). Panels G-J: Human CD34 progenitors were enriched from 10 primary NSγ transplant recipients to perform secondary transplantation assays. Initially, lineage-committed murine cells were depleted using immunomagnetic beads (not shown). After that depletion, the resulting fractions were enriched with human CD45+ cells that contained high percentages of CD45+ CD34neg cells (G). The progenitor cells were further enriched after the depletion of mature human cells. Human CD19+ and CD3+ cells were removed entirely (not shown). The CD34+ cells were CD45dim as expected for progenitors (panel H). Human hematopoiesis was established in secondary NSγ recipients at 6 (I) or 12 weeks (J) after transplantation.
Complete hematopoietic development in NSγ mice. After long-term transplantation assays (≥ 19 weeks), human hematopoietic engraftment was analyzed in 32 NSγ mice. In total, 27 mice had detectable human hematopoietic cells, and the levels of chimerism were dose dependent (A). Complete hematopoietic development was documented in 16 NSγ mice, as defined by human T-cell development in multiple tissues. These mice exhibited high levels of chimerism and were predominately mice that had been transplanted with high doses of Linneg ALDHbr CD34+ cells (B). The human CD45+ cells within the bone marrow of these mice included CD19+ B cells (C), CD3+ T cells (C-E), CD56+ NK cells (E), CD13+ myeloid cells, and CD34+ progenitors (F). The CD3+ cells coexpressed canonical T-cell antigens (CD8; D). Panels G-J: Human CD34 progenitors were enriched from 10 primary NSγ transplant recipients to perform secondary transplantation assays. Initially, lineage-committed murine cells were depleted using immunomagnetic beads (not shown). After that depletion, the resulting fractions were enriched with human CD45+ cells that contained high percentages of CD45+ CD34neg cells (G). The progenitor cells were further enriched after the depletion of mature human cells. Human CD19+ and CD3+ cells were removed entirely (not shown). The CD34+ cells were CD45dim as expected for progenitors (panel H). Human hematopoiesis was established in secondary NSγ recipients at 6 (I) or 12 weeks (J) after transplantation.
To test whether these mice maintained human hematopoietic stem cells, retransplantation assays were performed in secondary NSγ recipients. CD34+ progenitors were enriched from the bone marrows of 10 mice that had demonstrated human T-cell development in long-term transplants (Figure 6G-H). In each case, the progenitors were derived from approximately 70% of the bone marrow from the primary NSγ mouse. Ultimately, each secondary NSγ recipient was transplanted with progenitors from a single mouse and received no more than 30 000 human CD34+ cells. At either 6 or 12 weeks after transplantation, human hematopoietic chimerism was noted in 20% of secondary recipients (Figure 6I-J). The levels of chimerism within secondary NSγ recipients were low (≤ 0.5%); however, these mice did exhibit multiple-lineage hematopoietic development consisting of human B-lymphoid and myeloid cells. In total, these data indicate that the mice that developed human T cells exhibited a complete range of in vivo hematopoietic development and maintained transplantable human progenitors. Based on these mice, long-term SRCs represented 1 in 4896 Linneg ALDHbr CD34+ cells (95% CI: 1/3660-1/6557).
Indications for hematopoietic progenitors with limited developmental capacities
As described above, at 19-21 weeks after transplantation, human hematopoietic development was noted within the bone marrow of 27 of 32 NSγ mice. Although 16 of these mice exhibited complete hematopoietic development, 11 mice exhibited various degrees of more limited hematopoietic development. Eight of these 11 mice had been transplanted with ≤ 1000 human progenitors and typically exhibited low levels of human hematopoietic chimerism. These data suggested that incomplete hematopoietic development was a common outcome in transplants initiated with low progenitor cell doses.
Of these 11 mice, 8 established multiple-lineage hematopoietic chimerism with myeloid and B-lymphoid development (supplemental Figure 2). None of these mice had detectable human T cells within their bone marrow. Curiously, 4 of these mice had detectable human T cells within either their spleen (3 mice) or thymus (1 mouse). The significance of tissue-specific T-cell development was unclear; therefore, for these analyses, the engraftment was characterized as “incomplete.” In addition, 3 of these 11 NSγ mice did not establish multiple-lineage hematopoietic chimerism, but did maintain a single human hematopoietic lineage for 19-21 weeks after transplantation. Two mice demonstrated very low-level human myeloid development within the bone marrow (0.5%-1%; supplemental Figure 2). In those mice, human lymphoid cells and CD34+ progenitors were not detected in the bone marrow. Furthermore, human hematopoietic cells were not detected within either their spleen or blood. Finally, one mouse demonstrated T-cell development that was exclusively detected within the thymus, with no human hematopoietic development noted in any other tissue (supplemental Figure 2).
One premise for establishing this model for CBT had been that Linneg ALDHbr CD34+ cells capture the total content of transplantable progenitors within a CB graft. To confirm this, 11 control mice were transplanted with either 3000 or 10 000 purified Linneg ALDHneg CD34+ cells. Human hematopoietic development was detectable within 2 of these mice in long-term transplantation assays; however, the levels of human chimerism within the bone marrow were very low (< 0.5%), and human hematopoietic development was incomplete (supplemental Figure 3). In one mouse, human myeloid and B cells were detectable within the marrow and spleen. In that mouse, human T cells were detected within the thymus, but not within the bone marrow. The second mouse exhibited single-lineage myeloid development. Therefore, even within the highly permissive NSγ xenograft, the chimerism established by Linneg ALDHneg CD34+ cells was weak, infrequent, and incomplete. These data confirmed previous studies that had indicated that ALDHneg cells were not a significant source of transplantable progenitors.23,24
Discussion
Factors that can increase the speed of hematopoietic engraftment and immune recovery after CBT would greatly improve the safety and availability of this therapy. Successful CBT has been associated with the progenitor content of individual CB grafts.6,7,10 Therefore, the current studies evaluated the impact that CB progenitors have on the pace and completeness of hematopoietic engraftment in NSγ xenograft transplants. These experiments were performed using Linneg ALDHbr CD34+ progenitors, which simultaneously enrich both short- and long-term SCID-repopulating cells and therefore represent the entire spectrum of transplantable CB progenitors.23-26 Accordingly, throughout all of these studies, these progenitors consistently established dose-dependent human hematopoietic chimerism in NSγ mice (Figure 7). To evaluate the impact of the progenitors themselves, the Linneg ALDHbr CD34+ progenitors were highly purified, and the transplants were performed in the absence of any mature CB cells that might interfere with, or enhance, hematopoietic reconstitution.
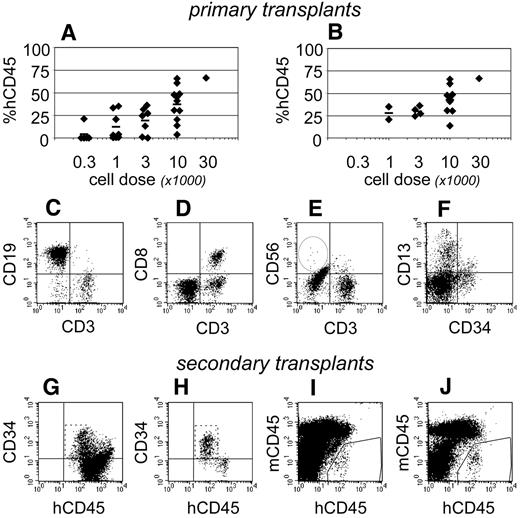
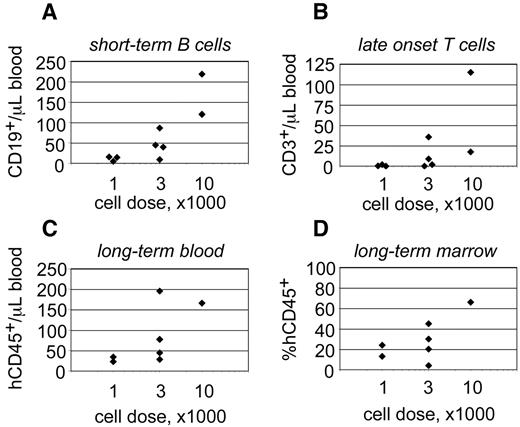
The behavior of progenitors isolated from single CB units. Linneg ALDHbr CD34+ progenitors were isolated from a single CB unit and transplanted into 9 NSγ mice at different cell doses. The peripheral blood of those mice was monitored for CD45+ cells 7 weeks after transplantation (A) and for CD45+ CD3+ T cells at 19 weeks after transplantation (B). Twenty-one weeks after transplantation, the mice were killed, and their levels of human hematopoietic chimerism was analyzed in both the peripheral blood (C) and bone marrow (D).
The behavior of progenitors isolated from single CB units. Linneg ALDHbr CD34+ progenitors were isolated from a single CB unit and transplanted into 9 NSγ mice at different cell doses. The peripheral blood of those mice was monitored for CD45+ cells 7 weeks after transplantation (A) and for CD45+ CD3+ T cells at 19 weeks after transplantation (B). Twenty-one weeks after transplantation, the mice were killed, and their levels of human hematopoietic chimerism was analyzed in both the peripheral blood (C) and bone marrow (D).
The current studies evaluated the CB progenitors in transplantation assays performed using NSγ mice.27-30 One key advantage to the NSγ xenograft model was that human hematopoietic chimerism could be evaluated in the peripheral blood, making it possible to monitor the progress of each transplant over time in much the same way that clinical transplants are monitored. In this way, progenitors that had been isolated from single CB units could be evaluated at multiple time points and within multiple different mice (Figure 7). Importantly, as early as 4 weeks after transplantation, the human CD45+ cells that were detectable within the blood corresponded with multiple-lineage human hematopoietic engraftment to the bone marrow.
A second key advantage to the NSγ model is that it supports the development of human T cells.27,28 In contrast, studies performed in other NOD/SCID strains evaluate human lymphocyte engraftment solely on the basis of B-cell development.23,35,36,38,42 In murine xenograft models, CB progenitors establish rapid B-cell engraftment that coincides with myeloid engraftment.35,38 Indeed, in the current studies, human B cells were detectable within the peripheral blood, spleen, and bone marrow as early as 4 weeks after transplantation. In contrast, in clinical CBT, the donor-derived B- and T-cell development are not rapid and do not correspond with the initial time to myeloid engraftment. Our studies in NSγ mice documented a late-onset human T-cell development that is more consistent with the significant, and critical, delays in lymphocyte development that accompany CBT.
The impact of progenitor cell dose
The primary effect of high progenitor cell doses was to produce robust, complete responses. This was evident in the time required to establish short-term human hematopoietic chimerism (Figure 2), the time required to establish T-cell development (Figure 4), and in the ability to establish complete, long-term hematopoietic development (Figure 6). Based on 16 mice that established complete human hematopoietic development, the frequency for long-term SRCs within the Linneg ALDHbr CD34+ fraction was 1 in 4896 cells (95% CI: 1/2767-1/8663). This estimate had very strong concordance with our previous studies, where long-term SRCs represented 1 in 4687 ALDHbr CD34+ cells (95% CI: 1/2180-1/10 077).23 The hallmark for this group was human lymphomyeloid development that included human CD3+ T cells within the bone marrow, spleen, and thymus. We note that this estimate excluded 8 mice that exhibited mixed-lineage lymphomyeloid engraftment that did not include T cells. These mice would have been scored as positive in other NOD/SCID xenograft models. However, given the efficiency of T-cell development in the NSγ xenografts, these 8 mice did not establish complete chimerism.
In mice that received the highest transplant cell doses (≥ 10 000 progenitors), human hematopoietic cells were reliably detected within the peripheral blood within 4 weeks. Similarly, in these same mice, human T cells could be detected within the peripheral blood within 13 weeks. In both cases, the chimerism was observed 3 weeks earlier than what was observed in mice that had been transplanted with lower progenitor cell doses. This high cell dose was essentially twice the estimate for the frequency of long-term SRCs. However, the SRC estimate is a mathematical tool that establishes a minimal number of progenitors that would be required to reliably achieve chimerism (ie, 37%). To achieve chimerism more consistently, a higher cell dose would be required, as was reflected in our studies by the fact that complete long-term chimerism was observed in 80% of the mice that were transplanted with 10 000 progenitors (Table 1). This same high cell dose was required to advance the initial time to engraftment. This was in contrast to the fact that, 7 weeks after transplantation, 1000 progenitors were sufficient to establish short-term chimerism in 75% of the mice (Table 1). This suggested that the kinetics of early engraftment are sensitive to the total content of long-term repopulating cells.
The most common consequences of low Linneg ALDHbr CD34+ progenitor cell doses was low levels of chimerism and incomplete hematopoietic development. Of 5 mice that were transplanted at the lowest cell dose (300 cells), none established complete human hematopoiesis; however, 2 mice exhibited limited human hematopoietic development. Similarly, of 8 mice that were transplanted with 1000 cells, only 2 exhibited complete human hematopoietic development. However, 4 additional mice established a limited, mixed-lineage hematopoietic development. In mice that demonstrated incomplete hematopoietic development, the human cells likely arose from progenitors that will eventually exhaust their capacity to contribute to hematopoiesis.12-14 Yet, their progeny were still detectable within long-term NSγ transplantation assays.
Human lymphocyte development and function in NSγ mice
Several key observations suggested that T-cell development within NSγ mice was derived from the active differentiation from Linneg ALDHbr CD34+ progenitors, rather than from the peripheral expansion of mature T cells. First, even after 19-21 weeks, the NSγ thymuses frequently contained immature human CD4+ CD8+ T-cell progenitors, and the thymic content of “double-positive” T cells coincided with the presence of human TRECs.39 Both of these parameters measure transient stages in early T-cell development. In contrast, at 19-21 weeks after transplantation, the human CD3+ cells within NSγ spleens had matured into CD4+ or CD8+ “single-positive” T cells. In addition, human TRECs were beneath the limits of detection within the spleens. Finally, the transplants were initiated using highly purified progenitor cell preparations that were derived from Linneg CB. These data indicated that early human T-cell development was supported in NSγ mice.
Although these data suggest that T-cell development is supported within the NSγ thymus, there are clear indications that human immune function is not established. The peripheral human T cells that were isolated from NSγ spleens did not respond to stimulation. Similarly, the human CD19+ B cells that were present within the NSγ spleens had differentiated into immature IgM+ IgD+ B cells; however, surface IgG was not detected. Furthermore, this fundamental phenotype remained unchanged between 12 weeks after transplantation and the end of long-term transplantation assays, some 7 weeks later. These data suggested that these cells initiated, but could not complete, B-cell maturation in NSγ mice.
The data presented here regarding lymphocyte development and function within the NSγ xenograft model were entirely consistent with that which has been described in NOG mice.40 Several experimental strategies might be used to enhance this model so that it is more reflective of immune reconstitution. In particular, lymphocyte function might be better supported if the NSγ mice received recombinant human IL7 at regular intervals.28 Alternatively, human immune function might be better developed if neonatal mice were transplanted shortly after birth by the direct injection of the progenitors into the liver.43 Finally, immune reconstitution might be more representative if the progenitors developed within human hematopoietic niches that can be provided by human fetal tissues.44
The NSγ xenograft transplant model has recently been adopted by multiple groups to address specific clinical concerns.31-33 Our studies provide evidence that the Linneg ALDHbr CD34+ progenitor cell dose influenced both the strength and the pace of hematopoietic chimerism within CBT. Our ongoing studies are currently using NSγ xenograft transplants as an experimental model to predict the effectiveness of clinical strategies where CB grafts might be augmented with purified ALDHbr progenitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Ms Lynn Martinek, of the Flow Cytometry Core Facility of the Duke Comprehensive Cancer Center, performed all fluorescence-activated cell sorting. Mr Jeff Hale, in the Immune Reconstitution and Biomarker Shared Resource Facility of the Duke Human Vaccine Institute, performed the TREC analyses. The authors gratefully acknowledge the helpful editorial comments provided by Drs R. Evans-Storms and A. Balber during the final preparation of this manuscript as well as helpful discussions with Dr J. Kurtzberg.
This work was supported through charitable donations received from Mrs Adelyn Luther (to R.W.S.). In addition, the Immune Monitoring Core of the Radiation Countermeasures Center of Research Excellence at Duke University received funding from the National Institutes of Health (U19-AI067798).
National Institutes of Health
Authorship
Contribution: C.L. contributed to collection of data, data analysis, and final approval of the manuscript; B.J.C. contributed to conception and design, data analysis, manuscript writing, and final approval of the manuscript; D.D. contributed to collection of data; G.D.S. contributed to collection of data and data analysis; N.J.C. contributed to conception and design and final approval of the manuscript; and R.W.S. contributed to conception and design, data analysis, manuscript writing, and final approval of the manuscript.
Conflict-of-interest disclosure: R.W.S. holds intellectual property regarding Aldefluor and holds stock in Aldagen Inc, the company that has licensed and commercialized this product. N.J.C. is a Founder of Aldagen Inc and holds stock in that company. Aldagen Inc did not contribute to the design or conduct of these studies and did not provide financial support. The remaining authors declare no relevant competing financial interests.
Correspondence: Robert W. Storms, Division of Cellular Therapy, Department of Medicine, Duke University Medical Center, Box 3361, Durham, NC 27710; e-mail: storms@duke.edu.