Abstract
We recently reported that Semaphorin 4D (Sema4D) and its receptors are expressed on the platelet surface and showed that Sema4D(−/−) mice have a selective defect in collagen-induced platelet aggregation and an impaired vascular injury response. Here we investigated the mechanisms involved, tested the role of platelet-platelet contacts in Sema4D-mediated events, and examined the relationship between Sema4D-dependent signaling and integrin αIIbβ3 outside-in signaling. The results show that spleen tyrosine kinase (Syk) activation, an early step in collagen signaling via the glycoprotein VI (GPVI)/FcRγ complex, is greatly reduced in Sema4D(−/−) platelets and can be restored by adding soluble Sema4D. Earlier events, including FcRγ phosphorylation, occur normally; later events are impaired. In contrast, when engagement of αIIbβ3 was blocked, Sema4D(−/−) and control platelets were indistinguishable in assays of Syk activation, adhesion, spreading on collagen, and activation of αIIbβ3. Finally, we found that, unlike the Sema4D knockout, αIIbβ3 blockade inhibited FcRγ phosphorylation and that stimulating aggregation with Mn2+ failed to normalize Syk activation in the absence of Sema4D. Collectively, these results show that αIIbβ3 and Sema4D jointly promote collagen responses by amplifying Syk activation, partly by forming integrin-mediated contacts that enable the binding of Sema4D to its receptors and partly through integrin outside-in signaling. These 2 processes are interdependent, but distinguishable.
Introduction
Semaphorins are a large family of proteins that were initially implicated in axonal guidance but have since been shown to contribute to organogenesis, vasculogenesis, angiogenesis and immune cell regulation.1 The semaphorin family includes more than 20 proteins divided into 8 classes. We have previously shown that platelets express on their surface the class-IV semaphorin, Sema4D (CD100), as well as its 2 known receptors, CD72 and Plexin-B1.2 We have also shown that platelets from Sema4D(−/−) mice have a selectively impaired response to collagen that produces a rightward shift in the aggregation dose/response curve when platelets are activated by either collagen or the glycoprotein VI (GPVI) collagen receptor agonist, convulxin.2 Notably, this is a collagen-specific defect, as platelet aggregation in response to other platelet agonists, including thromboxane A2 (TxA2), adenosine diphosphate (ADP) and a proteinase-activated receptor 4 (PAR4) agonist peptide, is normal. Sema4D(−/−) mice also have impaired responses in 3 models of vascular injury performed in vivo, reduced susceptibility to atherosclerosis when crossed into a low-density lipoprotein (LDL)–receptor knockout background, and are partially protected against the sensitizing effects of dyslipidemia, suggesting that Sema4D on the surface of platelets plays a biologically relevant role.2,3
Sema4D is a type I transmembrane protein that forms a disulfide-linked homodimer consisting of two 150-kDa subunits. It is present on the surface of resting platelets. However, additional copies of Sema4D are recruited to the surface upon platelet activation before being gradually cleaved and shed from the platelet surface by A disintegrin and metallopeptidase domain 17 (ADAM17).2 Of the 2 best known cell surface Sema4D receptors, Plexin-B1 is expressed on neurons4 and endothelial cells,5 where it mediates cytoskeletal changes via Ras, Rho and phospholipase Cγ.6-8 CD72 is a Sema4D receptor expressed on the surface of B cells. It has a cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM) that recruits Src homology phosphatase-1 (SHP-1) after B-cell antigen receptor activation, thus acting as an inhibitor of B-cell antigen receptor signaling.9 Engagement of CD72 with Sema4D on T cells relieves this inhibition by inducing the dissociation of the SHP-1/CD72 complex and the subsequent inactivation of SHP-1.10 Similarly, we have shown in platelets that the addition of recombinant soluble Sema4D dissociates SHP-1 from CD72.2
These observations suggest a model in which Sema4D on the platelet surface binds to receptors on adjacent platelets after integrin αIIbβ3–mediated formation of stable platelet-platelet contacts. Subsequent signaling via Sema4D and/or its receptors then promotes platelet activation in a contact-dependent manner. If this hypothesis is correct, it would account for the loss of function we have observed in the Sema4D(−/−) mice in vitro and in vivo.2 It would also bolster the general idea that platelets communicate with each other in a contact-dependent manner once aggregation begins and provide a basis for understanding the role of Sema4D and its receptors in platelets. Here we have tested this model and sought the underlying mechanisms by which Sema4D promotes collagen responses in platelets, focusing on events downstream of the principal collagen signaling receptor, GPVI, and its immunoreceptor tyrosine-based activation motif (ITAM)–containing partner, FcRγ.
The results show that collagen-induced activation of the spleen tyrosine kinase, Syk, an early event in collagen signaling, is diminished in Sema4D(−/−) platelets compared with Sema4D(+/+) platelets. Addition of recombinant soluble Sema4D exodomain restores collagen-induced Syk phosphorylation to wild-type levels during collagen-induced platelet aggregation. In contrast, when contacts are blocked, Syk phosphorylation falls to equally low levels in Sema4D(−/−) and Sema4D(+/+) platelets. The defect in Syk activation in Sema4D(−/−) platelets impairs phospholipase Cγ2 (PLCγ2) activation and reduces the rise in cytosolic Ca2+ that otherwise accompanies platelet activation by collagen. Earlier events in the collagen receptor GPVI pathway, including phosphorylation of FcRγ, occur normally in the Sema4D knockout.
Collectively, these observations emphasize the importance of contact-dependent signaling in maximizing platelet responses to collagen and suggest that once integrin-dependent contacts begin to form between platelets, Sema4D-mediated feedback reinforces Syk activation downstream of platelet collagen receptors. Hence, integrin blockade inhibits Syk activation in part by reducing integrin outside-in signaling and in part by preventing the binding of Sema4D to its receptors. Although Sema4D-mediated signaling is dependent on αIIbβ3 engagement, it is distinct from outside-in signaling by the integrin, which may explain why the Syk phosphorylation defect and resulting aggregation defects in Sema4D(−/−) platelets are limited to collagen and other GPVI agonists.
Methods
Materials
Antibodies recognizing phosphotyrosine (4G10P) and FcRγ were from Millipore, and an anti-Syk antibody (N19) was from Santa Cruz Biotechnology. The phospho-PLCγ2 (Y1217) antibody and phospho-Syk (Y519/520) were from Cell Signaling Technology, and anti-Sema4D (A8) was from BD Biosciences. Fura-2-acetoxymethyl ester (Fura 2-AM) was from Invitrogen. Fibrillar Type I collagen was obtained from Chrono-log. Acid-soluble collagen was from Inamed Biomaterials (PureCol) and convulxin from Alexis Biochemicals. AYPGQV was obtained from Biopeptide Co. Fibrinogen was obtained from Enzyme Research. Apyrase, aspirin, and prostaglandin E1 (PGE1) were obtained from Sigma-Aldrich, and Integrilin (eptifibatide, Schering-Plough) was obtained from the pharmacy of the Hospital of the University of Pennsylvania. Lec3.2.8.1 Chinese hamster ovary (CHO) cells stably transfected with hSema4D(1-657) containing a C-terminal polyhistidine (His) tag11 were kindly provided by Luca Tamagnone (University of Torino) and E. Yvonne Jones (University of Oxford). TALON metal affinity resin used to purify recombinant Sema4D was obtained from Clontech.
Mice
Sema4D(−/−) mice were described previously2,12 and have been backcrossed onto a C57 BL/6 background for at least 10 generations. Comparisons were made between Sema4D(+/+) and Sema4D(−/−) mice obtained from heterozygous crosses. Animal studies were carried out in compliance with University of Pennsylvania Institutional Animal Care and Use Committee approved protocols.
Isolation of human platelets
Blood obtained from healthy volunteers in accordance with the University of Pennsylvania Institutional Review Board was anticoagulated with acid-citrate-dextrose buffer (ACD; 65mM sodium citrate, 70mM citric acid, 100mM dextrose, pH 4.4) at a final ratio of 1:5 (blood:ACD) and centrifuged at 150g for 20 minutes to obtain platelet-rich plasma. Washed platelets were prepared by sedimentation at 340g for 15 minutes. Platelets were washed with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–EDTA (ethylenediaminetetraacetic acid)–NaCl (HEN) buffer (150mM NaCl, 1mM Na2EDTA, 10mM HEPES, pH 6.5) containing 1μM PGE1 and 1 U/mL apyrase and resuspended in modified Tyrode buffer (137mM NaCl, 20mM HEPES, 5.6mM glucose, 1 g/L bovine serum albumin [BSA], 1mM MgCl2, 2.7mM KCl, 3.3mM NaH2PO4, pH 7.4).
Isolation of mouse platelets
Blood was collected from the inferior vena cava of anesthetized mice (100 mg/kg pentobarbital) using a heparinized syringe (15 U/mL blood), diluted (1:1) with HEPES-Tyrode buffer and spun at 150g for 7 minutes. Platelet-rich plasma was isolated and washed with HEN buffer containing PGE1 (1μM) by centrifugation at 340g for 12 minutes followed by resuspension in modified Tyrode buffer.
Cytosolic calcium concentration
Mouse platelets were isolated and washed as described above (see “Isolation of mouse platelets”) and incubated with 10 μg/mL Fura-2-AM in Tyrode buffer for 45 minutes at room temperature. Platelets were then washed once with HEN buffer and resuspended in HEPES-Tyrode buffer at a concentration of 5 × 107 platelets/mL. Changes in cytosolic Ca2+ were detected under stirring conditions using an Aminco-Bowman Series 2 luminescence spectrometer with excitation at 340 and 380 nm and emission at 510 nm.
Immunoprecipitation and immunoblotting
Platelets were lysed with NP-40 buffer (1% NP-40, 50mM Tris [hydroxymethyl(aminomethane)], 150mM NaCl, 1mM EDTA) containing protease (Sigma-Aldrich) and phosphatase inhibitors (Calbiochem). After centrifugation at 16 000 × g for 15 minutes at 4°C, supernatants were incubated with antibody overnight at 4°C. Protein/antibody complexes were isolated with protein G agarose (Invitrogen) for 2 hours at 4°C. After 3 washes with lysis buffer, the beads were boiled in sample buffer (2% sodium dodecyl sulfate [SDS], 1% β-mercaptoethanol, 0.008% bromophenol blue, 80mM Tris pH 6.8, 1mM EDTA) and the proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% milk, probed as indicated, visualized by enhanced chemiluminescence (GE Healthcare) and quantified using ImageJ 1.42q software (National Institutes of Health). When antibody 4G10P was used, 3% bovine serum albumin was used in the blocking buffer instead of milk.
Flow cytometry
Platelet-rich plasma obtained from mouse whole blood was diluted with Tyrode buffer to a final concentration of 1 × 107 platelets/mL. Alexa Fluor 488–labeled fibrinogen (100 μg/mL, Invitrogen) was added to 100 μL of platelets with or without convulxin and incubated at 37°C for 10 minutes. Samples were fixed with 1% paraformaldehyde for 15 minutes at room temperature and washed with phosphate-buffered saline (PBS) before analysis on a FACSort flow cytometer (BD Biosciences).
Flow chamber
Studies were performed as previously described using a microfluidic flow chamber device.13 Each channel had a cross-sectional area of 80 μm (height) times 100 μm (width) and was positioned perpendicular to a 100-μm strip coated with acid soluble type I collagen (0.3 mg/mL). Whole mouse blood was anticoagulated with 93μM PPACK (H-(D)-Phe-Pro-Arg-chloromethylketone, Calbiochem), labeled with Alexa Fluor 488–conjugated CD41 monoclonal antibody (10 μg/mL final concentration, BD Biosciences) and then perfused through the chamber at an average wall shear rate of 800 second−1 for 5 minutes. When indicated, αIIb blocking antibody, Leo.H4 (EMFRET Analytics), was added at a final concentration of 25 μg/mL before perfusion. Real-time fluorescence images of the dynamics of platelet adhesion and aggregation within the chamber were observed using a Nikon Eclipse TE2000-U inverted microscope and captured using a Hamamatsu Digital Camera C9300 coupled to SlideBook 4.2 image acquisition software (Intelligent Imaging Innovations).
Platelet spreading
Blood was collected from Sema4D(+/+) and Sema4D(−/−) mice as described above (see “Isolation of mouse platelets”) and platelet-rich plasma was prepared. Glass coverslips (no. 1.5) were coated with 0.1 mg/mL acid soluble type I collagen (PureCol) overnight at 4°C and then rinsed with phosphate-buffered saline. Platelet spreading was visualized by reflection interference contrast microscopy (RICM) using an inverted microscope (Axiovert 200, Carl Zeiss) with an antiflex 63× oil-immersion objective and appropriate polarizers. Images were recorded using a charge-coupled device camera (Retiga Exi Fast Cooled Mono 12-bit camera 32-0082B-128 QImaging) at 1 frame/s. The contact area was measured as described.14
Statistical analysis
Statistical analysis was performed using a 2-tailed Student t test.
Results
Syk activation and subsequent downstream events are diminished when Sema4D is blocked or genetically deleted
Because Sema4D(−/−) platelets have a selective defect in collagen-induced aggregation, we focused our studies on events downstream of the principal collagen receptor, GPVI, beginning with measurements of the increase in cytosolic Ca2+ that normally accompanies platelet activation by GPVI agonists. This increase in cytosolic Ca2+ occurs due to clustering of GPVI leading to phosphorylation of FcRγ by Src family kinases, followed by the binding of Syk to FcRγ, activation of Syk, formation of a SLP-76 (Src homology 2 domain-containing leukocyte protein of 76 kDa) and Linker for activation of T cells (LAT)–dependent signaling complex, activation of PLCγ2 and production of 1,4,5-IP3, leading to Ca2+ release from the dense tubular system.15
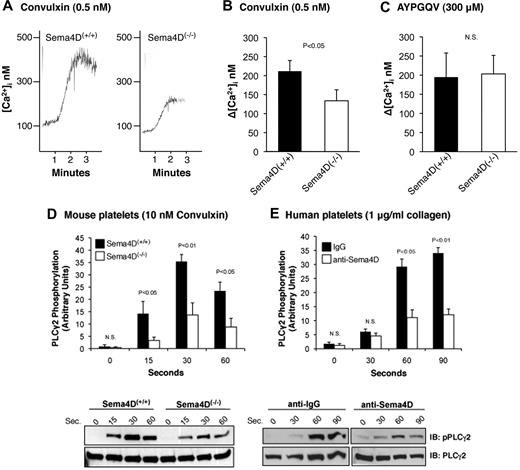
The data in Figure 1 show that the increase in Ca2+ initiated by convulxin, a snake venom lectin that activates GPVI, observed in Sema4D(−/−) platelets was about half that observed in platelets from matched Sema4D(+/+) controls. In contrast, the Ca2+ response to the PAR4 (thrombin receptor) agonist peptide, AYPGQV, was the same in Sema4D(+/+) and Sema4D(−/−) platelets. Convulxin-induced phosphorylation of Y1217 in PLCγ2 (a surrogate marker for activation) was also impaired in platelets from the Sema4D(−/−) mice relative to Sema4D(+/+) controls (Figure 1D), as was collagen-induced phosphorylation of PLCγ2 in human platelets preincubated with an antibody to Sema4D relative to an immunoglobulin G (IgG) control (Figure 1E).
The Ca2+ response and phosphorylation of PLCγ2 after convulxin stimulation is decreased in Sema4D(−/−) platelets. (A) Platelets from matched Sema4D(+/+) and Sema4D(−/−) mice were loaded with Fura-2 and then stimulated with 0.5nM convulxin (CVX). (B) Summary data (mean ± SEM) from 6 studies with CVX. (C) Summary data from platelets stimulated with the PAR4 agonist peptide, AYPGQV (300μM, mean ± SEM, N = 6). N.S. = not significant. (D) Platelets from matched Sema4D(+/+) and Sema4D(−/−) mice were stimulated with 10nM CVX. Phosphorylation of PLCγ2 was measured at the times indicated. A representative immunoblot is shown and the results of 5 experiments are summarized (mean ± SEM). (E) Human platelets preincubated for 15 minutes with either a Sema4D blocking antibody (10 μg/mL) or an immunoglobulin control (10 μg/mL) were stimulated with 1 μg/mL collagen (mean ± SEM, N = 3). N.S. = not significant.
The Ca2+ response and phosphorylation of PLCγ2 after convulxin stimulation is decreased in Sema4D(−/−) platelets. (A) Platelets from matched Sema4D(+/+) and Sema4D(−/−) mice were loaded with Fura-2 and then stimulated with 0.5nM convulxin (CVX). (B) Summary data (mean ± SEM) from 6 studies with CVX. (C) Summary data from platelets stimulated with the PAR4 agonist peptide, AYPGQV (300μM, mean ± SEM, N = 6). N.S. = not significant. (D) Platelets from matched Sema4D(+/+) and Sema4D(−/−) mice were stimulated with 10nM CVX. Phosphorylation of PLCγ2 was measured at the times indicated. A representative immunoblot is shown and the results of 5 experiments are summarized (mean ± SEM). (E) Human platelets preincubated for 15 minutes with either a Sema4D blocking antibody (10 μg/mL) or an immunoglobulin control (10 μg/mL) were stimulated with 1 μg/mL collagen (mean ± SEM, N = 3). N.S. = not significant.
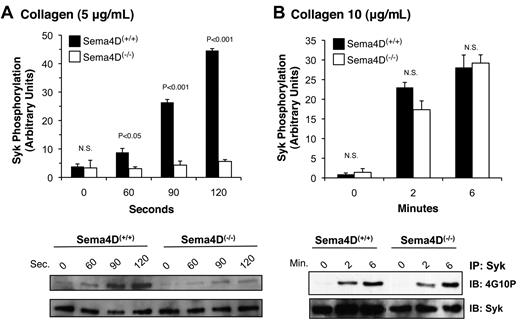
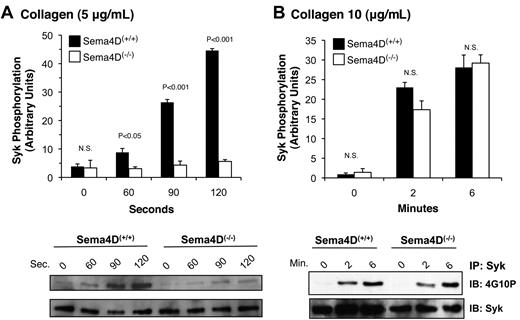
As already noted, PLCγ2 is activated by collagen or convulxin in platelets through a cascade of signaling proteins that includes the tyrosine kinase, Syk.16 After collagen stimulation, considerably less Syk phosphorylation was seen in Sema4D(−/−) platelets than in matched Sema4D(+/+) controls (Figure 2A). Increasing the collagen concentration overcame this difference (Figure 2B), just as increasing the collagen concentration overcomes the defect in platelet aggregation.2 In contrast, Syk phosphorylation in response to a PAR4 agonist peptide, like the rise in cytosolic Ca2+, was unaffected by the absence of Sema4D (data not shown). Thus, in Sema4D(−/−) platelets, there appears to be an agonist-selective defect in Syk activation, leading to the observed defects in PLCγ2 activation and intracellular Ca2+ release when platelets are activated by collagen.
Decreased Syk phosphorylation in Sema4D(−/−) platelets. Platelets from matched Sema4D(+/+) and Sema4D(−/−) mice were stimulated with either (A) 5 μg/mL or (B) 10 μg/mL collagen. Lysates were prepared and immunoprecipitated with anti-Syk followed by immunoblotting with the anti-phosphotyrosine antibody, 4G10P (mean ± SEM, N = 3). N.S. = not significant.
Decreased Syk phosphorylation in Sema4D(−/−) platelets. Platelets from matched Sema4D(+/+) and Sema4D(−/−) mice were stimulated with either (A) 5 μg/mL or (B) 10 μg/mL collagen. Lysates were prepared and immunoprecipitated with anti-Syk followed by immunoblotting with the anti-phosphotyrosine antibody, 4G10P (mean ± SEM, N = 3). N.S. = not significant.
Signaling events proximal to Syk activation occur normally in Sema4D(−/−) platelets
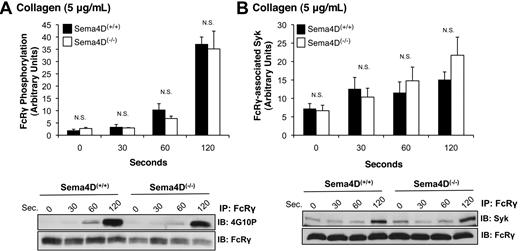
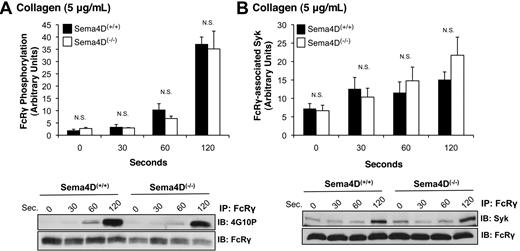
In collagen-stimulated platelets, Syk normally becomes autophosphorylated and activated after binding to the phosphorylated ITAM domain within FcRγ complexed with GPVI.17 FcRγ phosphorylation is mediated by the Src family kinases Fyn and Lyn, one or both of which are constitutively bound to GPVI.18,19 Because Syk phosphorylation was impaired in Sema4D(−/−) platelets, we next asked whether either FcRγ phosphorylation or the binding of Syk to phosphorylated FcRγ is also affected. Two approaches were used to measure FcRγ phosphorylation, both of which gave the same results. In the first, FcRγ was immunoprecipitated with anti-FcRγ antibody, and phosphorylated FcRγ was visualized with the anti-phosphotyrosine antibody, 4G10P (Figure 3A). In the second method, platelet lysates were probed directly with 4G10P, which detects a band corresponding to phosphorylated FcRγ at approximately 12 kDa (not shown). In both cases, the extent of FcRγ phosphorylation was the same in Sema4D(+/+) and Sema4D(−/−) platelets. There was also no difference between Sema4D(+/+) and Sema4D(−/−) platelets in the amount of Syk bound to FcRγ after collagen stimulation (Figure 3B). In Sema4D(+/+) platelets, the time course of FcRγ phosphorylation, association of Syk with FcRγ, and Syk phosphorylation were similar, all 3 events steadily increasing over the 120-second observation period. Only Syk phosphorylation in the Sema4D(−/−) deviated from the norm. Thus, the data suggest that the defect in collagen-induced Syk activation observed in the absence of Sema4D occurs after the binding of Syk to phosphorylated FcRγ.
FcRγ phosphorylation and the formation of the FcRγ/Syk complex proceed normally in the absence of Sema4D. Platelets from Sema4D(+/+) and Sema4D(−/−) mice were stimulated with collagen (5 μg/mL). (A) Lysates were immunoprecipitated with anti-FcRγ, followed by immunoblotting with the anti-phosphotyrosine antibody, 4G10P (mean ± SEM, N = 3). (B) Lysates were immunoprecipitated with anti-FcRγ followed by immunoblotting with anti-Syk (mean ± SEM, N = 6). N.S. = not significant.
FcRγ phosphorylation and the formation of the FcRγ/Syk complex proceed normally in the absence of Sema4D. Platelets from Sema4D(+/+) and Sema4D(−/−) mice were stimulated with collagen (5 μg/mL). (A) Lysates were immunoprecipitated with anti-FcRγ, followed by immunoblotting with the anti-phosphotyrosine antibody, 4G10P (mean ± SEM, N = 3). (B) Lysates were immunoprecipitated with anti-FcRγ followed by immunoblotting with anti-Syk (mean ± SEM, N = 6). N.S. = not significant.
Stable platelet-platelet contacts are needed for optimal activation of Syk
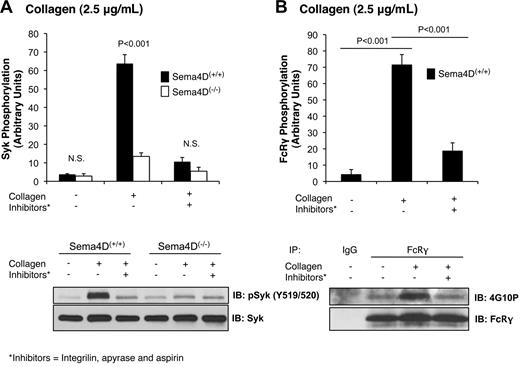
In the immune system, Sema4D mediates contact-dependent signaling between T cells and B cells by binding in trans to its cell surface receptor, CD72.10 We have proposed that Sema4D functions in a similar contact-dependent manner in platelets.2 In each of the experiments presented thus far, contacts between platelets were facilitated by stirring the platelets and allowing aggregation to occur. To test our hypothesis that Sema4D-mediated events are dependent on platelet contacts, we next examined collagen-induced Syk phosphorylation when aggregation was blocked by adding the αIIbβ3 antagonist, Integrilin (eptifibatide). Aspirin and apyrase were also added to block the contributions of released thromboxane and adenosine diphosphate, respectively. This combination of inhibitors was sufficient to block collagen-induced aggregation completely (data not shown). It also greatly reduced Syk phosphorylation in Sema4D(+/+) platelets and eliminated the difference between Sema4D(+/+) and Sema4D(−/−) platelets in both Syk (Figure 4A) and PLCγ2 (data not shown) phosphorylation. The level of Syk phosphorylation in Sema4D(+/+) platelets pretreated with inhibitors was similar to the level observed in Sema4D(−/−) platelets when no inhibitors were present. Similar results were obtained when contacts were limited by stimulating the platelets with collagen without stirring or when Integrilin was added without aspirin and apyrase (data not shown), suggesting the decrease in Syk phosphorylation is due to a failure to form stable platelet-platelet contacts.
Phosphorylation of Syk and FcRγ is diminished when platelet-platelet contacts are prevented. Platelets from Sema4D(−/−) and matched Sema4D(+/+) mice were incubated with Integrilin (10μM) plus aspirin (1mM) and apyrase (2 U/mL) for 30 minutes followed by collagen (2.5 μg/mL) for 2 minutes. (A) Immunoblot with anti-pSyk Y519/520 is shown. (B) FcRγ phosphorylation in Sema4D(+/+) platelets was detected by immunoprecipitating with anti-FcRγ followed by immunoblotting with anti-phosphotyrosine antibody, 4G10P. In both panels A and B, a representative experiment is shown and the results of 3 experiments are summarized (mean ± SEM). N.S. = not significant.
Phosphorylation of Syk and FcRγ is diminished when platelet-platelet contacts are prevented. Platelets from Sema4D(−/−) and matched Sema4D(+/+) mice were incubated with Integrilin (10μM) plus aspirin (1mM) and apyrase (2 U/mL) for 30 minutes followed by collagen (2.5 μg/mL) for 2 minutes. (A) Immunoblot with anti-pSyk Y519/520 is shown. (B) FcRγ phosphorylation in Sema4D(+/+) platelets was detected by immunoprecipitating with anti-FcRγ followed by immunoblotting with anti-phosphotyrosine antibody, 4G10P. In both panels A and B, a representative experiment is shown and the results of 3 experiments are summarized (mean ± SEM). N.S. = not significant.
In theory, preventing platelet-platelet contacts would not only block Sema4D-dependent signaling but also outside-in signaling through αIIbβ3, making the 2 processes difficult to distinguish if they have the same effects. However, we have observed that blocking αIIbβ3 either with the combination of aspirin, apyrase and Integrilin (Figure 4B), or with Integrilin alone (data not shown) also inhibits FcRγ phosphorylation (Figure 4B), while blocking Sema4D (Sema4D(−/−) platelets) does not (Figure 3A). In other words, FcRγ phosphorylation is regulated in part by the formation of contacts, but not by Sema4D-dependent signaling, while Syk phosphorylation is affected by both. This notable difference suggests that αIIbβ3-dependent outside-in signaling and Sema4D-dependent signaling pathways are distinct.
Integrin engagement promotes Syk phosphorylation in response to collagen
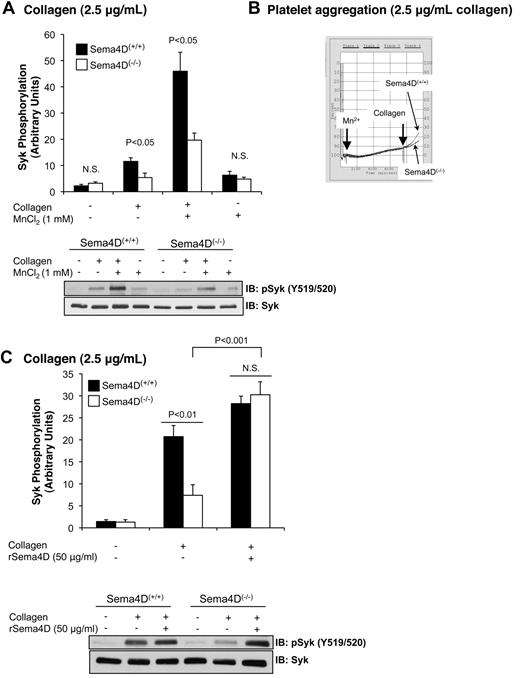
Platelet aggregation occurs when multivalent adhesive proteins such as fibrinogen bind to activated αIIbβ3 on the surface of adjacent platelets. Normally this occurs as a consequence of agonist-initiated inside-out signaling within the platelets. However, the activated conformation of integrins can also be promoted by adding Mn2+, even in the absence of an agonist.20-22 Here we observed that the addition of Mn2+ and fibrinogen without an agonist to resting platelets under stirring conditions was sufficient to cause a small increase in Syk phosphorylation (P < .05) that was similar in Sema4D(+/+) and Sema4D(−/−) platelets (Figure 5A). Under these conditions, both types of platelets were aggregated to the same minimal extent (Figure 5B). When collagen was added after 7 minutes with Mn2+, the extent of aggregation increased (Figure 5B). The combination of collagen and Mn2+ also caused a large increase in Syk phosphorylation (Figure 5A), as well as PLCγ2 phosphorylation (data not shown), but did not overcome the defect in Sema4D(−/−) platelets (Figure 5A). This suggests that achieving maximal Syk phosphorylation in collagen-stimulated platelets requires both Sema4D-dependent and integrin-dependent events. As a further test of this idea, we added recombinant Sema4D exodomain (rSema4D) as a soluble homodimer11 to Sema4D(+/+) and Sema4D(−/−) platelets and measured collagen-induced Syk phosphorylation under aggregating conditions. Addition of the Sema4D exodomain to resting platelets did not affect Syk phosphorylation (data not shown); however, after collagen stimulation, it restored Syk phosphorylation in Sema4D(−/−) to levels observed in wild-type platelets (Figure 5C). It also increased collagen-induced platelet aggregation (data not shown).
Promoting integrin engagement and Sema4D-mediated interactions increased Syk activation platelets. Washed platelets from Sema4D(+/+) and Sema4D(−/−) mice were supplemented with fibrinogen (150 μg/mL) and CaCl2 (1mM). Where indicated, MnCl2 (1mM) was added and the platelets were stirred for 7 minutes followed by a 2-minute incubation with or without collagen (2.5 μg/mL). (A) A representative immunoblot with anti-pSyk Y519/520 is shown, and the results of 4 experiments are summarized (mean ± SEM). (B) Representative aggregation traces for Mn2+-treated samples are shown. N.S. = not significant. (C) Washed platelets from Sema4D(+/+) and Sema4D(−/−) mice were incubated with 50 μg/mL rSema4D (recombinant Sema4D exodomain) for 10 minutes followed by collagen (2.5 μg/mL) for 2 minutes. A representative immunoblot with anti-pSyk Y519/520 is shown and results of 3 experiments are summarized (mean ± SEM). N.S. = not significant.
Promoting integrin engagement and Sema4D-mediated interactions increased Syk activation platelets. Washed platelets from Sema4D(+/+) and Sema4D(−/−) mice were supplemented with fibrinogen (150 μg/mL) and CaCl2 (1mM). Where indicated, MnCl2 (1mM) was added and the platelets were stirred for 7 minutes followed by a 2-minute incubation with or without collagen (2.5 μg/mL). (A) A representative immunoblot with anti-pSyk Y519/520 is shown, and the results of 4 experiments are summarized (mean ± SEM). (B) Representative aggregation traces for Mn2+-treated samples are shown. N.S. = not significant. (C) Washed platelets from Sema4D(+/+) and Sema4D(−/−) mice were incubated with 50 μg/mL rSema4D (recombinant Sema4D exodomain) for 10 minutes followed by collagen (2.5 μg/mL) for 2 minutes. A representative immunoblot with anti-pSyk Y519/520 is shown and results of 3 experiments are summarized (mean ± SEM). N.S. = not significant.
Collagen-induced activation of individual platelets proceeds normally in the absence of Sema4D
To further test our model that the contribution of Sema4D to platelet activation is contact dependent, we compared the behavior of Sema4D(+/+) and Sema4D(−/−) platelets in 3 assays performed under conditions in which platelet-platelet contacts were prevented or did not occur: αIIbβ3 activation, spreading on collagen under static conditions, and adhesion to collagen under flow.
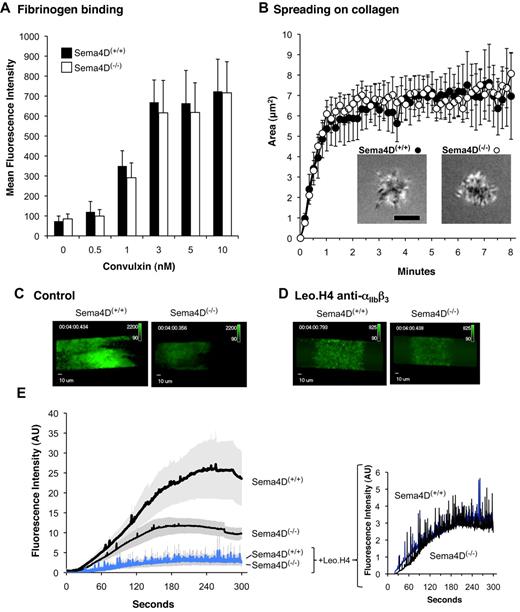
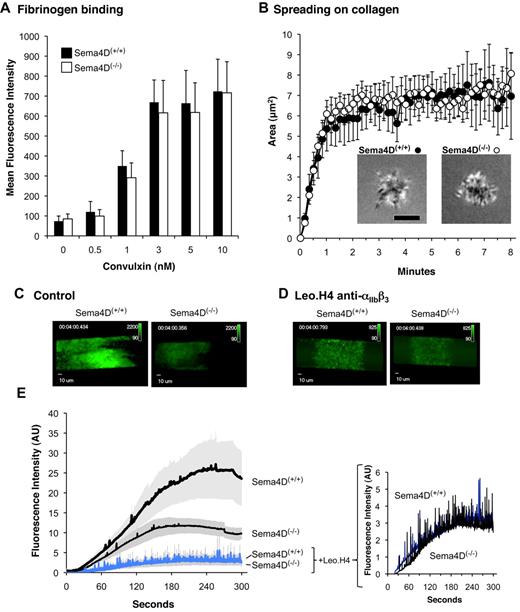
In the first assay, αIIbβ3 activation was detected using labeled fibrinogen after addition of convulxin to cluster GPVI. In these experiments, dilute platelets were used without stirring to minimize platelet-platelet contacts. The results show that over a range of convulxin concentrations, the extent of integrin activation (fibrinogen binding) was the same in Sema4D(+/+) and Sema4D(−/−) platelets (Figure 6A). In the second assay, dilute Sema4D(+/+) and Sema4D(−/−) platelets were allowed to adhere and spread on immobilized collagen under static conditions. The adhesion and spreading of isolated individual platelets was observed in real time using reflection interference contrast microscopy (RICM) and recorded digitally. Area measurements of these platelets showed no differences between Sema4D(+/+) and Sema4D(−/−) platelets in either the rate or the extent of spreading (Figure 6B).
Fibrinogen binding, spreading on collagen and the formation of an initial platelet monolayer on a collagen-coated surface under flow proceeds normally in Sema4D(−/−) platelets. (A) Fibrinogen binding. Platelets from Sema4D(+/+) and Sema4D(−/−) mice were incubated with Alexa Fluor 488–labeled fibrinogen (100 μg/mL) and convulxin (CVX). Fibrinogen binding was measured by flow cytometry (mean ± SEM, N = 4-8). (B) Spreading of individual platelets on collagen. Platelets from Sema4D(+/+) and Sema4D(−/−) mice were deposited on glass coverslips coated with acid soluble type I collagen (0.1 mg/mL). Platelet spreading was quantified by reflection interference contrast microscopy (RICM). Representative images are shown and the results of 3 experiments are summarized (mean ± SEM). Scale bar equals 3 μm. (C-E) Platelets in PPACK-treated whole blood obtained from Sema4D(−/−) and matched Sema4D(+/+) mice were labeled with Alexa Fluor 488–conjugated anti-CD41 (αIIb) and perfused through a microfluidic flow chamber at 800 second−1. Platelet accumulation was detected in real time. Where indicated, a second, anti-CD41 antibody (Leo.H4, unlabeled) was used to block αIIbβ3 and prevent platelet aggregates from forming. (C-D) Video captures after 4 minutes of platelet accumulation. Fluorescence intensities for Sema4D(+/+) and Sema4D(−/−) platelets have been adjusted equally for presentation. (E) Changes in fluorescence intensity over time as platelets accumulated on the collagen-coated surface (mean ± SEM, N = 4). See supplemental videos.
Fibrinogen binding, spreading on collagen and the formation of an initial platelet monolayer on a collagen-coated surface under flow proceeds normally in Sema4D(−/−) platelets. (A) Fibrinogen binding. Platelets from Sema4D(+/+) and Sema4D(−/−) mice were incubated with Alexa Fluor 488–labeled fibrinogen (100 μg/mL) and convulxin (CVX). Fibrinogen binding was measured by flow cytometry (mean ± SEM, N = 4-8). (B) Spreading of individual platelets on collagen. Platelets from Sema4D(+/+) and Sema4D(−/−) mice were deposited on glass coverslips coated with acid soluble type I collagen (0.1 mg/mL). Platelet spreading was quantified by reflection interference contrast microscopy (RICM). Representative images are shown and the results of 3 experiments are summarized (mean ± SEM). Scale bar equals 3 μm. (C-E) Platelets in PPACK-treated whole blood obtained from Sema4D(−/−) and matched Sema4D(+/+) mice were labeled with Alexa Fluor 488–conjugated anti-CD41 (αIIb) and perfused through a microfluidic flow chamber at 800 second−1. Platelet accumulation was detected in real time. Where indicated, a second, anti-CD41 antibody (Leo.H4, unlabeled) was used to block αIIbβ3 and prevent platelet aggregates from forming. (C-D) Video captures after 4 minutes of platelet accumulation. Fluorescence intensities for Sema4D(+/+) and Sema4D(−/−) platelets have been adjusted equally for presentation. (E) Changes in fluorescence intensity over time as platelets accumulated on the collagen-coated surface (mean ± SEM, N = 4). See supplemental videos.
Finally, we perfused whole blood anticoagulated with PPACK (H-(D)-Phe-Pro-Arg-chloromethylketone) over a collagen-coated surface in a microfluidics flow chamber at arterial shear rates (800 second−1).13 Under these conditions, an initial monolayer of platelets adhered to the collagen, after which additional platelets accumulated on top of those that arrived first, forming platelet-platelet contacts. The rate and extent of platelet accumulation was reduced by half in the absence of Sema4D, reflecting a role for Sema4D in producing optimal platelet activation on collagen (Figure 6C-E, and supplemental Videos available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The flow studies were then repeated after blocking platelet-platelet contacts with antibody Leo.H4, which prevents fibrinogen binding to mouse αIIbβ3. Blocking the integrin largely eliminated the formation of platelet aggregates, leaving little more than a monolayer of platelets. Under these conditions, there was no significant difference in the extent of accumulation of Sema4D(+/+) and Sema4D(−/−) platelets (P = .7 at 4 minutes, Figure 6D-E and supplemental Videos). Thus, the results of all 3 assays suggest that the loss of Sema4D affects platelet behavior only in settings in which platelet-platelet contacts are allowed to form.
Discussion
Formation of a hemostatic plug after vascular injury typically begins with the local generation of thrombin and exposure of collagen, followed by the capture of moving platelets by collagen and the subsequent accumulation of additional platelets. Once activated, platelets come in persistent contact with each other, allowing molecules on the surface of adjacent platelets to interact in trans and modulate thrombus growth and stability.23 The interaction of cell surface ephrin B1 and its receptors, Eph A4 and Eph B1, is one example of contact-dependent signaling that we have described previously.24 We have proposed that Sema4D and its receptors provide another.2 Here we investigated the mechanisms involved in Sema4D action, tested the role of contacts in Sema4D-mediated events, and examined the relationship between Sema4D-dependent signaling and integrin-mediated outside-in signaling.
The results showed that one of the early steps in GPVI signaling, phosphorylation of Syk, is greatly reduced in Sema4D(−/−) platelets. Earlier steps, including phosphorylation of the ITAM motif in the cytoplasmic domain of FcRγ by Src family kinases, occur normally, as does Syk binding to phosphorylated FcRγ. As a result of the Syk activation defect, downstream events were also affected, which led to decreased PLCγ2 activation and a diminished increase in the cytosolic Ca2+ concentration. Raising the collagen concentration or adding soluble Sema4D exodomain dimers overcame the defect in Syk phosphorylation, just as it overcame the aggregation defect in Sema4D(−/−) platelets.2
Notably, loss of Sema4D expression only affected platelet function when the studies were performed under conditions in which stable platelet-platelet contacts were allowed to form. When αIIbβ3 engagement was blocked or discouraged, individual Sema4D(−/−) platelets were indistinguishable from wild type in terms of the (much diminished) extent of Syk activation, the formation of a platelet monolayer under flow on a collagen-coated surface, and spreading on collagen under static conditions. Activation of αIIbβ3 in single platelet suspensions was also normal in Sema4D(−/−) platelets. Thus, Sema4D appears to promote collagen-induced platelet activation by increasing Syk activation in a contact-dependent manner after Syk binds to phosphorylated FcRγ. How this might occur is discussed below (see “A model for Sema4D in platelets”).
Integrin outside-in signaling versus Sema4D-mediated signaling
The relationship between outside-in signaling and Sema4D-dependent signaling is complex. The binding of Sema4D and other cell surface ligands to their receptors on adjacent cells can only occur when integrin engagement stabilizes platelet-platelet contacts. Previous studies have shown that outside-in signaling through αIIbβ3 also promotes Syk phosphorylation.25,26 Therefore, it might seem difficult to distinguish outside-in signaling from Sema4D-mediated signaling. However, we found that stimulating integrin engagement and outside-in signaling by adding Mn2+ in combination with collagen increased Syk phosphorylation without eliminating the difference between Sema4D(+/+) and Sema4D(−/−) platelets, while adding soluble Sema4D boosted collagen-stimulated in Sema4D(−/−) platelets to the same level as in Sema4D(+/+) platelets. We also found that inhibiting integrin engagement impaired FcRγ phosphorylation, which was not affected in the Sema4D knockout. Thus, Sema4D signaling and integrin outside-in signaling are intertwined, but distinguishable. In other words, while Sema4D signaling in platelets depends on prior integrin engagement, Sema4D makes its own contribution at a distinct point in the collagen signaling pathway once contacts have formed. This may be part of the explanation for why the aggregation defect in Sema4D(−/−) platelets is limited to collagen and other GPVI agonists.
A model for Sema4D in platelets
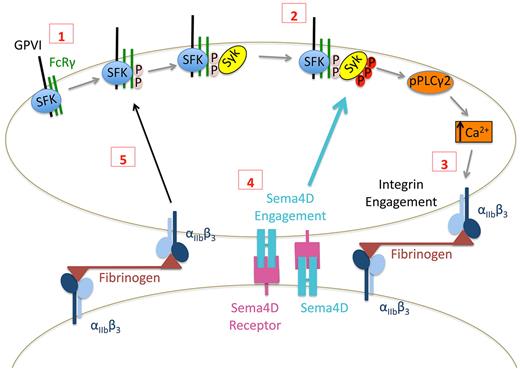
Taken together, these observations suggest a model in which Sema4D functions in a contact-dependent signaling pathway that reinforces Syk phosphorylation downstream of GPVI as illustrated in Figure 7. Although adhesion and the initial responses of platelets to collagen can occur normally in the absence of Sema4D, once platelets begin to form stable integrin-mediated contacts with other platelets, Sema4D can bind to its receptors. Along with integrin outside-in–mediated signaling, this amplifies collagen responses by increasing Syk activation and PLCγ2 activity. In vitro, the defect in Syk activation in the absence of Sema4D can be overcome by increasing the collagen concentration. This may be due to increased GPVI clustering and enhanced initial signaling that bypasses the need for the Sema4D-mediated boost in signaling. The decrease in platelet accumulation after laser or rose Bengal injury in Sema4D(−/−) cremaster muscle arterioles and the prolonged time to occlusion in carotid arteries exposed to FeCl3 that we have described previously all argue for the relevance in vivo of platelet Sema4D.2,3
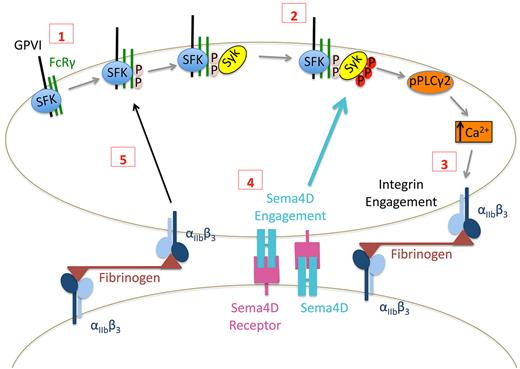
Proposed contact-dependent role of Sema4D in GPVI signaling. (1) Based on work by others, clustering of GPVI leads to Src family kinase (SFK)–mediated phosphorylation of FcRγ resulting in the recruitment and (2) subsequent phosphorylation of Syk. Subsequent signaling through phospholipase Cγ leads to integrin activation and (3) the formation of stable, integrin-dependent contacts between platelets. (4) The data presented here suggest that this allows Sema4D to engage in trans with its receptors, amplifying Syk activation. It also allows outside-in signaling by the integrin to promote Syk phosphorylation, in part by increasing FcRγ phosphorylation (5).
Proposed contact-dependent role of Sema4D in GPVI signaling. (1) Based on work by others, clustering of GPVI leads to Src family kinase (SFK)–mediated phosphorylation of FcRγ resulting in the recruitment and (2) subsequent phosphorylation of Syk. Subsequent signaling through phospholipase Cγ leads to integrin activation and (3) the formation of stable, integrin-dependent contacts between platelets. (4) The data presented here suggest that this allows Sema4D to engage in trans with its receptors, amplifying Syk activation. It also allows outside-in signaling by the integrin to promote Syk phosphorylation, in part by increasing FcRγ phosphorylation (5).
In theory, Sema4D could contribute to Syk phosphorylation by either promoting phosphorylation or inhibiting dephosphorylation. CD7210 and Plexin-B14 are the best characterized receptors for Sema4D and we have previously reported the expression of both these receptors in human platelets.2 We have proposed that either or both of these receptors are involved in Sema4D signaling in platelets as they are in other cell types, but definitive evidence is still lacking. We have also shown previously that CD72 is associated with SHP-1 in resting human platelets and that addition of either soluble Sema4D or a platelet agonist causes dissociation of the CD72/SHP-1 complex,2 similar to events in B cells.9,10 However, neither the CD72 knockout nor the plexin-B1 knockout alone appear to phenocopy the effects of the Sema4D knockout (H.J. and L.F.B., unpublished observations, December 2007). Therefore, it remains to be established whether Sema4D engages either of these receptors or some other receptor in trans when aggregation begins. It is also formally possible that signaling mediated by Sema4D occurs in a retrograde fashion when it engages receptors on nearby platelets. Such signaling is known to occur when ephrins engage with Eph kinases in platelets, and has also been shown for another semaphorin family member, Sema6D, during cardiac developement.27 Evidence in T cells has suggested the possibility of signaling downstream of Sema4D.28,29
In conclusion, we have shown that Sema4D contributes toward collagen signaling in platelets, and we propose that Sema4D plays a key role in the contact-dependent reinforcement of Syk activation downstream of GPVI. We have also provided evidence that contacts between platelets are important for Sema4D-mediated events, as is the case when T cells expressing Sema4D come in contact with B cells expressing CD72.30 Much still remains to be learned about the role of Sema4D in platelets. The absence of gross bleeding when Sema4D is genetically deleted in mice suggests that Sema4D is not essential for normal hemostasis. However, the observation that the loss of Sema4D expression impairs platelet responses to vascular injury,2 reduces platelet hyperactivity associated with dyslipidemia, and confers protection against the development of atherosclerosis3 suggests that Sema4D plays a larger role in response to pathological injury. Future studies will aim to further understand how Sema4D functions to promote collagen signaling in platelets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mark Kahn for critically reading the manuscript and Ms Aminat Saliu for her contributions to the PLCγ2 studies.
This work was supported by National Institutes of Health (NIH) P50-HL81012 (L.F.B.), NIH R33-HL087385 (D.A.H., L.F.B., and S.L.D.), and an award from the American Heart Association, 10SDG2610066 (K.B.N.). T.J.S. and D.L. were supported by American Heart Association postdoctoral fellowships 0525630U and 09POST2140195, respectively. L.Z. was supported by a grant from the Natural Science Foundation of China (NSFC; 80170410). K.M.W. and S.M. were supported by NIH training grant T32-HL007971.
National Institutes of Health
Authorship
Contribution: K.M.W. designed the study, performed experiments, analyzed the data, and wrote the paper; L.Z. designed the study, performed experiments, analyzed the data, and wrote the paper; H.J. performed experiments and analyzed the data; K.P.F. performed experiments and analyzed the data; T.J.S. designed the study; D.L. performed experiments and analyzed the data; A.N.T. performed experiments; K.B.N. provided reagents/analytical tools; S.M. provided reagents/analytical tools; A.K. provided reagents/analytical tools; H.K. provided reagents/analytical tools; D.A.H. provided reagents/analytical tools; S.L.D. provided reagents/analytical tools; and L.F.B. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Li Zhu, Soochow University, Rm 509, Bldg 703, 199 Ren'ai Rd, Suzhou Industrial Park, Suzhou, Jiangsu, China 215123; e-mail: zhul@suda.edu.cn; or Lawrence F. Brass, University of Pennsylvania, 915 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: brass@mail.med.upenn.edu.
References
Author notes
K.M.W. and L.Z. contributed equally to this work and are co–first authors.