Abstract
Because primary myelofibrosis (PMF) originates at the level of the pluripotent hematopoietic stem cell (HSC), we examined the effects of various therapeutic agents on the in vitro and in vivo behavior of PMF CD34+ cells. Treatment of PMF CD34+ cells with chromatin-modifying agents (CMAs) but not hydroxyurea, Janus kinase 2 (JAK2) inhibitors, or low doses of interferon-α led to the generation of greater numbers of CD34+ chemokine (C-X-C motif) receptor (CXCR)4+ cells, which were capable of migrating in response to chemokine (C-X-C motif) ligand (CXCL)12 and resulted in a reduction in the proportion of hematopoietic progenitor cells (HPCs) that were JAK2V617F+. Furthermore, sequential treatment of PMF CD34+ cells but not normal CD34+ cells with decitabine (5-aza-2′-deoxycytidine [5azaD]), followed by suberoylanilide hydroxamic acid (SAHA; 5azaD/SAHA), or trichostatin A (5azaD/TSA) resulted in a higher degree of apoptosis. Two to 6 months after the transplantation of CMAs treated JAK2V617F+ PMF CD34+ cells into nonobese diabetic/severe combined immunodeficient (SCID)/IL-2Rγnull mice, the percentage of JAK2V617F/JAK2total in human CD45+ marrow cells was dramatically reduced. These findings suggest that both PMF HPCs, short-term and long-term SCID repopulating cells (SRCs), are JAK2V617F+ and that JAK2V617F+ HPCs and SRCs can be eliminated by sequential treatment with CMAs. Sequential treatment with CMAs, therefore, represents a possible effective means of treating PMF at the level of the malignant SRC.
Introduction
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm (MPN), which is thought to originate at the level of a pluripotent hematopoietic stem cell (HSC).1-5 A gain-of-function mutation JAK2V617F has been identified in the MPNs, which is present in the granulocytes of approximately 95% of patients with polycythemia vera and 50% of patients with either PMF or essential thrombocythemia. In approximately 10% of patients with JAK2V617F-negative PMF, an additional somatic mutation of the thrombopoietin receptor gene MPL has also been identified.6 Furthermore, malignant clones harboring additional genetic abnormalities including the TET oncogene family member 2 (TET2), the additional sex combs like gene and the gene for isocitrate dehydrogenase 1 as well as characteristic cytogenetic abnormalities have been observed in PMF indicating that multiple genetic events are likely responsible for the origins of this MPN.7
Epigenetic modifications leading to the dysregulation of critical genes that contribute to cell proliferation, differentiation, cell death, and trafficking have also been thought to play a role in the origins of PMF.8,9 Mutations in TET2, for instance, might contribute to the origins of PMF by altering chromatin structure. TET1 affects the conversion of 5-methylcytosine to 5-hydroxymethylcytosine and therefore influences epigenetic regulation of transcription.10 Previously we had reported that the constitutive mobilization of cluster of differentiation (CD)34+ cells in PMF could be accounted by in part by the reduced expression of chemokine (C-X-C motif) receptor (CXCR)4 by PMF CD34+ cells which has been attributed to hypermethylation of its promoter.11 In addition, we have reported that the sequential treatment of PMF CD34+ cells with a DNA methyltransferase inhibitor, 5-aza-2′-deoxycytidine (5azaD), followed by an histone deacetylase (HDAC) inhibitor (HDACI), trichostatin A (TSA), resulted in an up-regulation of CXCR4 expression by PMF CD34+ cells leading to correction of the abnormal cellular trafficking characteristic of PMF as well as a reduction of the burden of malignant hematopoietic progenitor cells (HPCs).12,13 These preclinical studies illustrate the profound effects of chromatin-modifying agents (CMAs) on PMF HPCs and led us to further evaluate these strategies, this time using drugs that are currently approved for the treatment of other hematologic malignancies, and to determine whether they affect malignant stem cells.
We hypothesize that curative drug therapies for PMF would ideally eliminate or at least reduce the burden of PMF HSCs/HPCs, allowing their normal counterparts to predominate. Currently, the standard surrogate assay for human HSCs assesses the ability of a putative HSC population to establish hematopoiesis in immunodeficient mice. In this report, we provide evidence that sequential treatment with 5azaD followed by the HDACI, suberoylanilide hydroxamic acid (SAHA), affects not only PMF HPCs but also stem cells. Sequential treatment with CMAs therefore represents a promising therapeutic strategy for the treatment of PMF patients.
Methods
Description of patients and cell isolation procedures
Peripheral blood (PB) was collected from 32 patients who fulfilled the World Health Organization diagnostic criteria for PMF.14 All patients gave signed informed consent as approved by the Institutional Review Board of the Mount Sinai School of Medicine and in accordance with the Declaration of Helsinki.
Granulocytes were isolated by previously described techniques.15 CD34+ cells were selected from PB low-density mononuclear cells from PMF patients and cord blood (CB) collections provided by the New York Blood Center using a CD34+ cell selection kit (StemCell Technologies). The purity of the CD34+ cell population was analyzed using a FACSCanto flow cytometer (BD Biosciences). CD34+ cell populations with a purity ≥ 90% were used in all experiments. Due to limitation of the number of cells available from the individual patient, cells from subsets of patients were used for particular experiments as outlined in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
JAK2V617F and MplW515L mutational analyses
JAK2V617F and MplW515L were detected by analyzing the PB granulocytes of patients with PMF by using a real-time quantitative polymerase chain reaction (PCR) assay using an allelic discrimination method as described previously.16 The JAK2V617F status and allele burden of each patient studied is provided in supplemental Table 1. Among the 32 PMF subjects, 18 patients were JAK2V617F-positive while none possessed MplW515L. The JAK2V617F-negative patients were further analyzed for marker chromosomal abnormalities using fluorescence in situ hybridization (FISH).17 Patient 32 was characterized by a marker chromosomal abnormality Del(13)(q13) that could be detected by FISH.
Treatment of CD34+ cells with various therapeutic agents
PMF CD34+ cells (1 × 105/mL) were cultured in Iscove modified Dulbecco medium (IMDM; Lonza) containing 30% fetal bovine serum (FBS; HyClone Laboratories) supplemented with 100 ng/mL stem cell factor (SCF), 100 ng/mL feline McDonough sarcoma-like tyrosine kinase 3 ligand (FL), 100 ng/mL thrombopoietin (TPO), and 50 ng/mL interleukin-3 (IL-3; Amgen) and incubated in a humidified incubator maintained at 37°C with 5% CO2. After an initial 16 hours of incubation, cells were either exposed to 5azaD (1μM; Teva Pharmachemie) for 48 hours, or SAHA (1μM, gift of Merck) for 6 days, or 1 of 2 JAK2 inhibitors, JAK inhibitor 1 (100nM; Calbiochem) or AZ1480 (100nM, gift of AstraZeneca), hydroxyurea (50μM; Sigma-Aldrich), or pegylated (Peg)–IFN-α2a (125 U; Roche) alone for 3 days. In addition, CD34+ cells were exposed to 5azaD at a concentration of 1μM for 48 hours, and then washed and distributed to new culture plates containing SCF, FL, and TPO with either SAHA (1μM) for an additional 6-day culture period, or TSA (16.5nM; Sigma-Aldrich) for a 7-day culture period.12,13 In addition, cultures containing cytokines alone were performed in parallel. In some experiments, the cultured PMF CD34+ cells were reisolated as described above after a period of culture and assayed for their ability to migrate in vitro in response to chemokine (C-X-C motif) ligand (CXCL)12 or form hematopoietic colonies in semisolid media.
To determine whether CMAs were able to affect normal hematopoietic stem/progenitor cells, CB CD34+ cells (1 × 105/mL) were also cultured and treated with CMAs in an identical fashion.
Flow cytometric analysis of CD34+ cells
Primary CD34+ cells and CD34+ cells isolated after incubation were labeled with anti–human (h)CD34 monoclonal antibody (mAb) conjugated to allophycocyanin (APC) and CXCR4 mAb (clone 12G5) conjugated to phycoerythrin (PE). All mAbs were purchased from BD Biosciences. Each analysis was paired with a corresponding matched isotype control. Immediately before flow cytometric analysis, 1 μg/mL of propidium iodide (PI; Sigma-Aldrich) was added to exclude nonviable cells. Cells were analyzed by flow cytometry, and at least 10 000 viable cells were acquired from each sample (BD FACSDiva software Version 6.1.2).
The percentage of CD34+ cells undergoing apoptosis was determined using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences). CD34+annexin V+PI− cells were regarded as cells undergoing apoptosis.
Migration assay
The migratory behavior of primary PMF CD34+ cells before and after culture was determined as previously described using 6.5-mm diameter, 5-μm pore Transwell plates (Corning).15,18 The percentage of cells migrating was calculated by determining the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment. Migrating cells were also assayed in semisolid media for their ability to form hematopoietic colonies and those colonies were plucked and their JAK2 status determined.19
Hematopoietic progenitor cell assays
Primary CD34+ cells or CD34+ cells reisolated after culture with cytokines or cytokines plus various agents were assayed in semisolid media as described previously.19 Briefly, 5 × 102 cells were plated in duplicate culture dishes containing 1 mL IMDM with 1.1% methylcellulose, 30% FBS, 5 × 10−5 mol/L 2-mercaptoethanol (StemCell Technologies), to which SCF, TPO, IL-3, IL-6, granulocyte macrophage colony-stimulating factor (GM-CSF), each at 100 ng/mL, and 5 U/mL erythropoietin (Amgen) were added. Colonies were enumerated after 12 to 14 days of incubation. Individual colonies were plucked and analyzed for the JAK2V617F using a nested allele-specific PCR as previously described19 and the percentage of JAK2V617F-positive colonies formed was determined.
NOD/SCID marrow repopulating cell assay
Nonobese diabetic (NOD)/severe combined immune deficiency (SCID)/IL2Rγnull mice were purchased from The Jackson Laboratory. All experiments were approved by the Animal Care Committee of the Mount Sinai School of Medicine. PMF CD34+ cells from 7 patients with a granulocyte JAK2V617F allele burden ranging from 35% to 86% were treated with 5azaD/SAHA or 5azaD/TSA and their cellular phenotype analyzed, their ability to form hematopoietic colonies in vitro and the JAK2V617F status of individual colonies determined. In addition, primary PMF CD34+ cells or the total number of cells generated after culture from the identical number of PMF CD34+ cells in the presence of cytokines alone or cytokines plus 5azaD/SAHA or 5azaD/TSA (0.4-20.6 × 105 CD34+ cells/mouse) were transplanted via the tail vein into 8- to 9-week-old sublethally irradiated (240 cGy) NOD/SCID/IL2Rγnull mice. Two, 4, or 6 months after transplantation, mice were killed and cells were recovered from the bone marrow (BM) of femurs, tibias, humeri, and spleens, and the PB of the recipient mice. The presence of hCD45+, hCD33+, hGlycophorin A+, hCD41a+, hCD19+, hCD3+, and hCD34+ cells was determined by mAb staining and flow cytometric analysis. Each analysis was paired with a corresponding matched isotype control. Cells obtained from mice not receiving human cell transplants were analyzed in a similar fashion in parallel to exclude the possibility of false positive immunostaining. The antibodies used did not crossreact with murine cells. hCD45+ cells in the BM of the recipient mice were further selected using CD45 MicroBeads (Miltenyi Biotec) and the purity of hCD45+ cells were ≥ 90%. The JAK2V617F/JAK2total percentage in the genomic DNA of selected hCD45+ cells was determined by real-time quantitative PCR using the allelic discrimination method which is capable of detecting as few as 0.25% mutant allele in 40 ng of DNA isolated from approximately 6000 human engrafted cells.16
Statistical analysis
The results are reported as the mean ± SD of data obtained from 4 to 6 individual experiments. Statistical significance was determined using Student t tests or paired-sample t tests. All P values were 2-sided.
Results
5azaD/SAHA treatment increases the numbers of PMF CD34+CXCR4+ cells
Bogani C et al have previously reported that a short-term in vitro treatment with 5-azaD reduced CXCR4 promoter methylation, increased membrane expression of CXCR4 and resulted in improved migration of CD34+ cells in response to CXCL12 in vitro.11 In addition we have shown that sequential treatment of PMF CD34+ cells with 5azaD/TSA, resulted in an up-regulation of CXCR4 expression by PMF CD34+ cells and correction of the abnormal cellular trafficking characteristic of PMF as well as a reduction of the burden of malignant HPCs.12,13 We, therefore, explored whether similar activity could be achieved with an HDACI currently approved for clinical use to develop a strategy for treating patients with PMF. SAHA is an HDACI currently approved to treat patients with cutaneous T-cell lymphoma (CTCL).20 We evaluated the effects of 5azaD or SAHA alone or the sequential treatment with these 2 CMAs on PMF CD34+ cells.
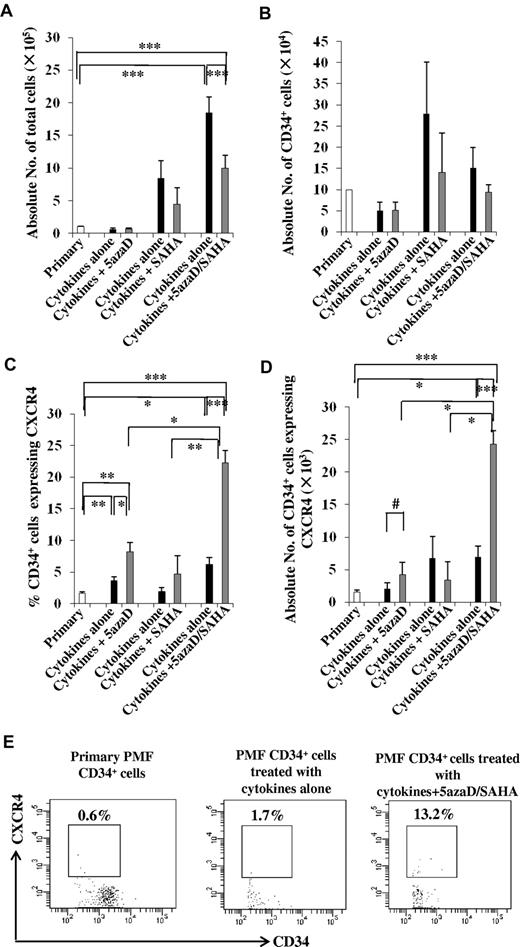
CD34+ cells were first treated with cytokines alone or cytokines plus 5azaD for 2 days and were phenotypically characterized. As shown in Figure 1A-B, a similar reduction in the total number of cells and CD34+ cells were observed in the cultures of incubated with cytokines alone or cytokines plus 5azaD alone. The percentage of CD34+ cells which expressed CXCR4 was, however, significantly greater (8.2% ± 1.5%) after 5azaD treatment than that achieved with cells exposed to cytokines alone (3.6 ± 0.6%, P < .05) or primary PMF CD34+ cells (1.6% ± 0.3%, P < .01; Figure 1C). The number of CD34+ cells which expressed CXCR4 in cultures containing cytokines plus 5azaD (4.3 ± 1.8 × 103) was 2-fold greater than that detected in cultures exposed to cytokines alone (2.1 ± 0.9 × 103, P = .08; Figure 1D).
Effects of CMAs on PMF CD34+ cells. PMF CD34+ cells were treated with cytokines plus 5azaD for 2 days (n = 6), cytokines plus SAHA for 6 days (n = 6), or cytokines plus 5azaD/SAHA for 8 days (n = 22). The cultures containing cytokines alone were performed in parallel. The cultured cells were then phenotypically characterized. (A) The total number of cells generated after the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines plus CMAs. ***P < .001. (B) The number of CD34+cells generated after the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines plus CMAs. The percentage (C) and number (D) of CD34+ cells expressing CXCR4 was significantly increased in the cultures containing cytokines plus 5azaD/SAHA compared with cultures containing cytokines plus 5azaD alone or plus SAHA alone. *P < .05; **P < .01; ***P < .001; #P = .08. (E) A representative flow cytometric pattern demonstrating the analysis of the percentage of PMF CD34+ cells which express CXCR4 after the culture PMF CD34+ cells in the presence of cytokines alone or cytokines plus 5azaD/SAHA.
Effects of CMAs on PMF CD34+ cells. PMF CD34+ cells were treated with cytokines plus 5azaD for 2 days (n = 6), cytokines plus SAHA for 6 days (n = 6), or cytokines plus 5azaD/SAHA for 8 days (n = 22). The cultures containing cytokines alone were performed in parallel. The cultured cells were then phenotypically characterized. (A) The total number of cells generated after the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines plus CMAs. ***P < .001. (B) The number of CD34+cells generated after the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines plus CMAs. The percentage (C) and number (D) of CD34+ cells expressing CXCR4 was significantly increased in the cultures containing cytokines plus 5azaD/SAHA compared with cultures containing cytokines plus 5azaD alone or plus SAHA alone. *P < .05; **P < .01; ***P < .001; #P = .08. (E) A representative flow cytometric pattern demonstrating the analysis of the percentage of PMF CD34+ cells which express CXCR4 after the culture PMF CD34+ cells in the presence of cytokines alone or cytokines plus 5azaD/SAHA.
We next examined the effect of SAHA treatment alone on PMF CD34+ cells. Incubation of CD34+ cells with cytokines alone resulted in a 5.8- to 10.2-fold increase, while treatment with the same cytokines and SAHA resulted in a 2- to 7-fold increase in the total number of cells compared with the number of input cells (P = .11, Figure 1A). The number of CD34+ cells in cultures containing cytokines alone (2.8 ± 1.2 × 105) was statistically similar to the number in cultures exposed to cytokines plus SAHA (1.4 ± 0.9 × 105, P = .37; Figure 1B), and the absolute number of CD34+ cells expressing CXCR4 in cultures containing cytokines plus SAHA (3.4 ± 2.8 × 103) was only half that observed in the cells exposed to cytokines alone (Figure 1D). These findings suggest that treatment with SAHA alone does not enhance the expression of CXCR4 by PMF CD34+ cells.
We next investigated the effects of the sequential treatment with 5azaD and SAHA on PMF CD34+ cells. PMF CD34+ cells cultured (Figure 1A) in the presence of cytokines plus 5azaD/SAHA generated more total cells (10.0 ± 1.9 × 105) than the number of primary cells used to initiate the cultures (1.1 ± 0.01 × 105, P < .001) but fewer cells than that observed in cultures containing cytokines alone (18.5 ± 2.4 × 105, P < .001). The absolute number of CD34+ cells in cultures exposed to cytokines alone (1.5 ± 0.5 × 105) was similar to that documented in cultures exposed to cytokines plus 5azaD/SAHA (0.9 ± 0.2 × 105; Figure 1B). The percentage of CD34+ cells which expressed CXCR4 was, however, significantly greater (22.3% ± 1.9%) after 5azaD/SAHA treatment than that achieved after exposure to cytokines alone (6.2% ± 1.1%, P < .001), primary PMF CD34+ cells (1.6% ± 0.3%, P < .001), or cells exposed to 5azaD alone (P < .05) or SAHA alone (P < .01; Figure 1C). The number of CD34+ cells that expressed CXCR4 in cultures containing cytokines plus 5azaD/SAHA was greater than that present in primary CD34+ cells (P < .001) or cells exposed to cytokines alone (P < .001), 5azaD alone (P < .05), or SAHA alone (P < .05) (Figure 1D). This effect of 5azaD/SAHA on CXCR4 expression was observed irrespective of the JAK2 mutational status of the patients studied (data not shown).
5azaD/SAHA treatment increases the migratory capacity of PMF CD34+ cells in response to CXCL12
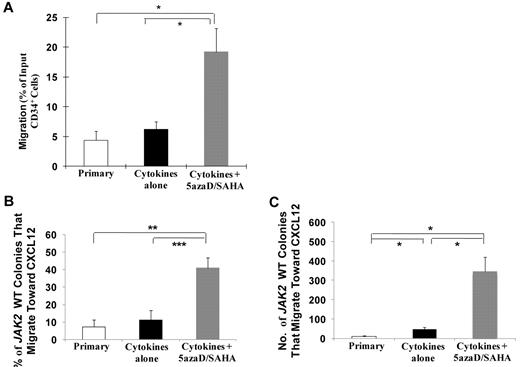
The reisolated 5azaD/SAHA treated PMF CD34+ cells had a greater capacity to migrate toward CXCL12 (19.2% ± 3.96%) compared with CD34+ cells exposed to cytokines alone (6.2% ± 1.3%, P < .05) or primary CD34+ cells (4.4% ± 1.6%, P < .05; Figure 2A).
5azaD/SAHA treatment increases the percentage of JAK2V617F wild-type PMF CD34+ cells that migrate toward CXCL12. (A) The migratory behavior of primary PMF CD34+ cells or PMF CD34+ cells reisolated after ex vivo culture in the presence of cytokines alone or cytokines plus 5azaD/SAHA was determined by the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment of a 6.5-mm diameter, 5-μm pore Transwell plate (n = 7). *P < .05. JAK2V617F-positive migrating cells were further assayed in semisolid media and analyzed for the JAK2V617F mutation using nested allele-specific PCR (n = 4). The percentage (B) and number (C) of JAK2V617F wild-type colonies (CFU-GM plus BFU-E plus CFU-Mixed) that migrate toward CXCL12 from cultures treated with cytokines plus 5azaD/SAHA was significantly greater than that observed from cultures exposed to cytokines alone or primary PMF CD34+ cells. *P < .05; **P < .01; ***P < .001.
5azaD/SAHA treatment increases the percentage of JAK2V617F wild-type PMF CD34+ cells that migrate toward CXCL12. (A) The migratory behavior of primary PMF CD34+ cells or PMF CD34+ cells reisolated after ex vivo culture in the presence of cytokines alone or cytokines plus 5azaD/SAHA was determined by the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment of a 6.5-mm diameter, 5-μm pore Transwell plate (n = 7). *P < .05. JAK2V617F-positive migrating cells were further assayed in semisolid media and analyzed for the JAK2V617F mutation using nested allele-specific PCR (n = 4). The percentage (B) and number (C) of JAK2V617F wild-type colonies (CFU-GM plus BFU-E plus CFU-Mixed) that migrate toward CXCL12 from cultures treated with cytokines plus 5azaD/SAHA was significantly greater than that observed from cultures exposed to cytokines alone or primary PMF CD34+ cells. *P < .05; **P < .01; ***P < .001.
The JAK2 mutational status of HPCs (colony forming unit [CFU]–granulocyte, monocyte [GM], burst forming unit erythroid [BFU-E], and CFU-Mixed) from 4 patients (JAK2V617F granulocyte allele burden range, 37%-77%) that migrated in response to CXCL12 was then analyzed. Both the percentage and total number of JAK2 wild-type colonies assayed from CD34+ cells treated with cytokines plus 5azaD/SAHA that had migrated was significantly greater (40.9% ± 5.8%; 347 ± 74.0) than JAK2 wild-type colonies assayed from CD34+ cells exposed to cytokines alone that had migrated (11.3% ± 5.3%, P < .001; 45 ± 11.4, P < .05) or primary PMF CD34+ cells that had migrated (7.2% ± 3.9%, P < .01; 9.8 ± 3.8, P < .05; Figure 2B-C).
5azaD/SAHA treatment reduces the percentage of malignant PMF HPCs
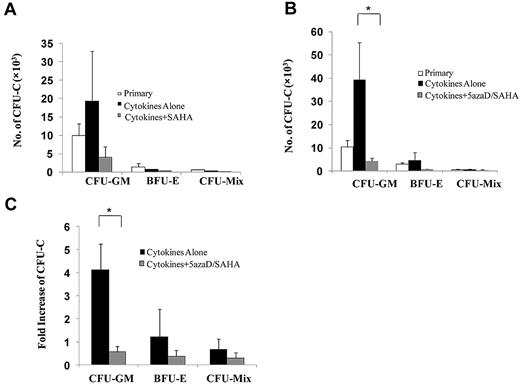
We further investigate the effect of SAHA alone or 5azaD/SAHA on PMF HPCs. As shown in Figure 3A, a similar number of HPCs were assayed from PMF CD34+ cells treated with SAHA as that assayed from CD34+ cells exposed to cytokines alone or primary PMF CD34+ cells. Individual colonies from 2 JAK2V617F-positive PMF patients (JAK2V617F allele burden of granulocytes was 60% and 77%) were plucked and analyzed for the JAK2V617F using a nested allele-specific PCR. As shown in Table 1, the treatment of PMF CD34+ cells with SAHA alone did not substantially reduce the percentage of JAK2V617F-positive colonies or JAK2V617F homozygous colonies.
5azaD/SAHA treatment reduces the number of assayable PMF HPCs. (A) Total number of colonies assayed from the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines alone or cytokines plus SAHA for 6 days (n = 6). (B) Total number of colonies assayed from the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines alone or cytokines plus 5azaD/SAHA for 8 days (n = 15). *P < .05. (C) The degree of the expansion of progenitor cells after ex vivo culture was determined by dividing the number of colonies assayed from cultures performed in the presence of cytokines alone or cytokines plus 5azaD/SAHA by that assayed from input primary CD34+ cells (n = 15). *P < .05.
5azaD/SAHA treatment reduces the number of assayable PMF HPCs. (A) Total number of colonies assayed from the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines alone or cytokines plus SAHA for 6 days (n = 6). (B) Total number of colonies assayed from the culture of PMF CD34+ cells (1 × 105) in the presence of cytokines alone or cytokines plus 5azaD/SAHA for 8 days (n = 15). *P < .05. (C) The degree of the expansion of progenitor cells after ex vivo culture was determined by dividing the number of colonies assayed from cultures performed in the presence of cytokines alone or cytokines plus 5azaD/SAHA by that assayed from input primary CD34+ cells (n = 15). *P < .05.
By contrast, 5azaD/SAHA treatment resulted in a marked reduction of all classes of assayable progenitor cells (CFU-GM, BFU-E, and CFU-Mixed), compared with PMF CD34+ cells exposed to cytokines alone (P < .05) or primary PMF CD34+ cells (P = .08; Figure 3B-C). The inhibitory effect of 5azaD/SAHA treatment on PMF HPCs was similar to that previously reported with 5azaD/TSA treatment.12
We further analyzed the JAK2V617F status of HPCs assayed from PMF CD34+ cells treated with 5azaD/SAHA from 6 different patients (JAK2V617F granulocyte allelic burden of ranging from 35%-80%). CD34+ cells were reisolated after treatment of cells from these same patients with cytokines alone or cytokines plus 5azaD/SAHA. As shown in Table 2, 5azaD/SAHA treatment resulted in not only a reduction in the proportion of JAK2V617F+ HPCs (53.7% ± 10.1%) compared with cells exposed to cytokines alone (81.8% ± 7.5%, P < .01) or primary PMF CD34+ cells (82.5% ± 8.3%, P < .01) but also a reduction in the proportion of JAK2V617F homozygous HPCs (13.4% ± 6.5%) compared with cells exposed to cytokines alone (33.4% ± 9.7%, P < .05) or primary PMF CD34+ cells (55.3% ± 15.1%, P = .09).
In addition, in a single case with JAK2V617F negative PMF but with a marker chromosomal abnormality Del(13)(q13), Patient 32, the effect of 5azaD/SAHA treatment on the malignant clone was examined by performing FISH analysis of the cells within colonies. Of the colonies cloned from primary CD34+ cells from this patient, 53.8% were composed of cells where > 90% contained the marker chromosomal abnormality compared with 46.2% in cultures exposed to cytokines alone, while only colonies containing cells lacking the chromosomal abnormality were observed in the culture exposed to cytokines plus 5azaD/SAHA. These data suggest that 5azaD/SAHA treatment can preferentially eliminate the malignant HPCs of PMF patients with genetic defects other than JAK2V617F.
5azaD/SAHA treatment does not affect normal CD34+ cells
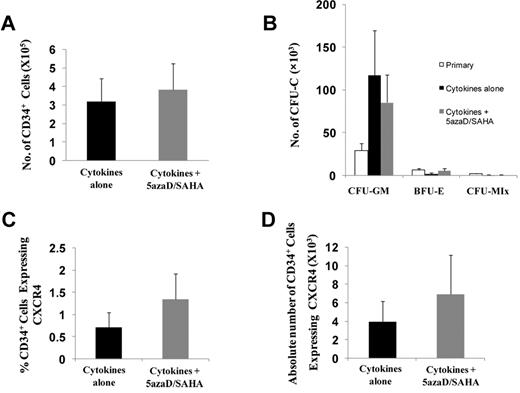
We next examined the effects of 5azaD/SAHA treatment on CB CD34+ cells. As shown in Figure 4A and B, the absolute number of CB CD34+ cells and assayable HPCs in cultures exposed to cytokines alone was similar to that detected in cultures exposed to cytokines plus 5azaD/SAHA. The percentage of cells within the 5azaD/SAHA-treated CB CD34+ cells which expressed CXCR4 was, however, 1.9 times greater than the cells exposed to cytokines alone (Figure 4C). In addition, treatment of CB CD34+ cells in vitro with cytokines plus 5azaD/SAHA resulted in a 1.8-fold increase in the absolute numbers of CD34+CXCR4+ cells compared with CD34+ cells exposed to cytokines alone (Figure 4D). These findings suggest that 5azaD/SAHA treatment at the doses used is not toxic to normal CD34+ cells but does result in up-regulated CXCR4.
5azaD/SAHA treatment does not reduce the number of HPCs assayed from CB. CB CD34+ cells (1 × 105; n = 6) were treated with 5azaD/SAHA for 8 days in the same way as PMF CD34+ cells. (A) The absolute number of CD34+ cells in the culture of CB CD34+ cells (1 × 105) treated with cytokines alone or cytokines plus 5azaD/SAHA. (B) Total number of colonies assayed from the culture of CB CD34+ cells (1 × 105) in the presence of cytokines alone or cytokines plus 5azaD/SAHA. (C-D) The percentage and total number of CD34+ cells which expressed CXCR4 in CB CD34+ cells treated with cytokines alone or cytokines plus 5azaD/SAHA.
5azaD/SAHA treatment does not reduce the number of HPCs assayed from CB. CB CD34+ cells (1 × 105; n = 6) were treated with 5azaD/SAHA for 8 days in the same way as PMF CD34+ cells. (A) The absolute number of CD34+ cells in the culture of CB CD34+ cells (1 × 105) treated with cytokines alone or cytokines plus 5azaD/SAHA. (B) Total number of colonies assayed from the culture of CB CD34+ cells (1 × 105) in the presence of cytokines alone or cytokines plus 5azaD/SAHA. (C-D) The percentage and total number of CD34+ cells which expressed CXCR4 in CB CD34+ cells treated with cytokines alone or cytokines plus 5azaD/SAHA.
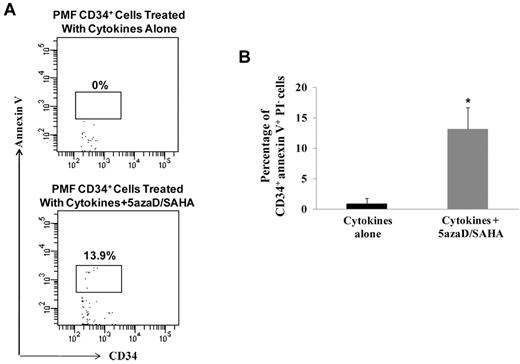
5azaD/SAHA treatment induces the apoptosis of PMF CD34+ cells
To explore additional mechanisms by which 5azaD/SAHA affects PMF CD34+ cells, we determined whether sequential treatment with CMAs was associated with an increased degree of apoptosis. As shown in Figure 5B, the percentage of CD34+annexin V+PI− cells were significantly greater in PMF CD34+ cells treated with cytokines plus 5azaD/SAHA (13.2% ± 3.5%) compared with that observed with PMF CD34+ cells treated with cytokines alone (0.9% ± 0.9%, P < .05). Remarkably treatment of CB CB34+ cells with the same doses of 5azaD/SAHA in the presence of cytokines was not associated with the same degree of apoptosis (0.4% ± 0.4%), indicating that PMF CD34+ cells are more sensitive than CB CD34+ cells to the actions of these CMAs.
5azaD/SAHA treatment induces the apoptosis of PMF CD34+ cells. (A) A representative flow cytometric pattern demonstrating the analysis of the percentage of PMF CD34+ cells which are annexin V+PI− after the culture of PMF CD34+ cells in the presence of cytokines alone or cytokines plus 5azaD/SAHA. (B) The percentage of CD34+annexin V+PI− cells were greater in PMF CD34+ cells treated with cytokines plus 5azaD/SAHA compared with that observed in PMF CD34+ cells treated with cytokines alone (n = 6). *P < .05.
5azaD/SAHA treatment induces the apoptosis of PMF CD34+ cells. (A) A representative flow cytometric pattern demonstrating the analysis of the percentage of PMF CD34+ cells which are annexin V+PI− after the culture of PMF CD34+ cells in the presence of cytokines alone or cytokines plus 5azaD/SAHA. (B) The percentage of CD34+annexin V+PI− cells were greater in PMF CD34+ cells treated with cytokines plus 5azaD/SAHA compared with that observed in PMF CD34+ cells treated with cytokines alone (n = 6). *P < .05.
Effects of JAK2 inhibitors, hydroxyurea, or Peg-IFN-α2a on PMF CD34+ cells
The current standard of care for PMF patients include hydroxyurea and IFN-α. Recently several small molecule inhibitors of JAK2 have been evaluated in this patient population.21 We compared the effects of 2 small JAK2 inhibitors (JAK inhibitor 1 and AZ1480), hydroxyurea, and Peg-IFN-α2a on PMF CD34+ cells to assess the uniqueness of the effects of 5azaD/SAHA on PMF CD34+ cells. As shown in Table 3, incubation of PMF CD34+ cells (1 × 105) with cytokines alone for 3 days generated greater numbers of total cells (2.8 ± 0.7 × 105) than in cultures performed in the presence of AZ1480 (1.7 ± 0.4 × 105, P < .05), hydroxyurea (0.7 ± 0.2 × 105, P < .05), or Peg-IFN-α2a (1.7 ± 0.5 × 105, P < .05), while cultures performed in the presence of JAK inhibitor 1 contained a similar number of total cells (2.2 ± 0.7 × 105). However, similar numbers of CD34+ cells were observed in cultures containing cytokines alone (0.6 ± 0.3 × 105) as cultures with cytokines plus each of the JAK2 inhibitors, hydroxyurea, or Peg-IFN-α2a (Table 3). Similarly treatment with none of these agents altered CXCR4 expression by CD34+ cells. A similar number of HPCs were assayed from CD34+ cells treated with cytokines plus each of these agents individually as that assayed from primary CD34+ cells or CD34+ cells exposed to cytokines alone. None of these agents substantially reduce the percentage of JAK2V617F+ colonies or JAK2V617F homozygous colonies (Table 4). These findings suggest that unlike 5azaD/SAHA, none of these agents was capable of eliminating JAK2V617F HPCs or up-regulating CXCR4 expression by PMF CD34+ cells.
Effects of sequential treatment with CMAs on PMF SCID repopulating cells
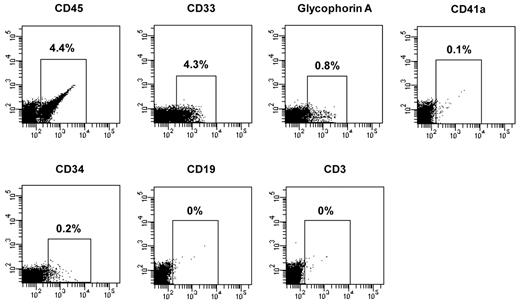
After transplantation, short-term SRCs are thought to contribute to hematopoiesis initially but their contribution to blood cell production persists for only a limited period of time, while long-term SRCs are thought to contribute to hematopoiesis after a delay but to be responsible for sustained blood cell production.22 These classes of SRCs can be distinguished by monitoring the kinetics of engraftment in immunodeficient mice. We therefore evaluated the effect of sequential treatment with CMAs on short-term SRCs assayed 2 months after transplantation and long-term SRCs, assayed 4 or 6 months after transplantation. CD34+ cells from 7 patients (JAK2V617F allele burden of granulocytes ranged from 35% to 86%) were transplanted and the JAK2V617F allele burden of hCD45+ cells was determined in the marrow of NOD/SCID/IL2Rγnull mice. Two months after transplantation, 4.4% of the marrow cells were hCD45+ cells in mice receiving primary CD34+ cells from Patient 4 compared with 0.2% in mice receiving cells treated with cytokines alone or cytokines and 5azaD/TSA. The donor cells receiving primary PMF CD34+ cells were composed of myeloid cells belonging to multiple hematopoietic lineages (CD33+ cells, 4.3%; glycophorin A+ cells, 0.8%; CD41a+ cells, 0.1%; and CD34+ cells, 0.2%) but not CD19+ or CD3+ cells (Patient 4, Figure 6). The percentage of JAK2V617F/JAK2total in hCD45+ cells from BM of the 2 mice transplanted with primary CD34+ cells from this patient was 71.5% and 34.7% in mice transplanted with cells treated with cytokines alone and 6.8% in mice transplanted with cells treated with 5azaD/TSA (Table 5, Patient 4).
Differentiative pattern of PMF CD34+ cells in NOD/SCID/IL2Rγnull mice 2 months after the transplantation. Two months after transplantation, the mouse transplanted with primary CD34+ cells of Patient 4 was killed and cells were recovered from the BM of femurs, tibias, and humeri of the recipient mice. The presence of human primary cells and various lineage cells in the BM of the recipient mice was determined by mAb staining and flow cytometric analysis.
Differentiative pattern of PMF CD34+ cells in NOD/SCID/IL2Rγnull mice 2 months after the transplantation. Two months after transplantation, the mouse transplanted with primary CD34+ cells of Patient 4 was killed and cells were recovered from the BM of femurs, tibias, and humeri of the recipient mice. The presence of human primary cells and various lineage cells in the BM of the recipient mice was determined by mAb staining and flow cytometric analysis.
Four months after transplantation similar numbers of hCD45+ cells (0.04%-0.1%) were detected in the BM of mice receiving either PMF CD34+ cells treated with cytokines alone or cytokines and 5azaD/TSA. A persistently marked reduction in the percentage of JAK2V617F/JAK2total in hCD45+ cells was, however, observed in the BM of mice transplanted with PMF CD34+ cells treated with 5azaD/TSA (22.5%, 8.8%, and 28.8%,) compared with that observed in mice transplanted with the corresponding CD34+ cells treated with cytokines alone (74.5%, 82.4%, and 79.9% in Patients 6, 9, and 10; Table 5).
Similarly, 6 months after transplantation, human marrow cell chimerism was also documented (Patients 1, 3, and 12, Table 5). The engrafted cells in the BM of these mice were composed of myeloid cells belonging to multiple hematopoietic lineages (CD33+, Glycophorin A+, CD41a+, CD19+, and CD34+ cells) but not CD3+ cells (Patient 12). The percentage of JAK2V617F/JAK2total in the gDNA of BM hCD45+ cells was 82.5%, 97.6%, and 92.8% in mice transplanted with primary CD34+ cells compared with 27.1%, 41.5%, and 94.9% in mice transplanted with CD34+ cells treated with cytokines alone and 3.6%, 7.4%, and 17.8% in mice transplanted with CD34+ cells treated with 5azaD/SAHA. A similar reduction of the percentage of JAK2V617F/JAK2total in hCD45+ BM cells of mice transplanted with CMAs treated CD34+ cells from an additional patient was also observed (Patient 6, Table 5). These findings suggest that both short-term and long-term PMF SRCs are affected by the malignant process and that various classes of JAK2V617F+ SRCs can each be eliminated by sequential treatment with CMAs.
Discussion
In the present report, we show that the in vitro treatment with sequential 5azaD/SAHA or 5azaD/TSA, but not 5azaD or SAHA alone, had a profound effect on PMF CD34+ cells, resulting in a dramatic reduction in the number of JAK2V617F+ HPCs. These effects were not observed after the treatment with other agents including JAK2 inhibitors, hydroxyurea, or Peg-IFN-α2a. We have chosen to use SAHA for these studies because clinical trials and animal models have shown that SAHA can induce growth arrest and death of cancer cells in vitro and in vivo, at concentrations that have little or no toxicity for normal cells.23-27 We have demonstrated that both short- and long-term SRCs in PMF are JAK2V617F+ and that JAK2V617F+ SRCs can be eliminated by sequential treatment with CMAs, leading to engraftment by SRCs with wild-type JAK2. The degree of engraftment by 5azaD/SAHA- or 5azaD/TSA-treated PMF CD34+ cells might be underestimated in the present report since fewer PMF CD34+ cells treated with 5azaD/SAHA or 5azaD/TSA were transplanted into each mice compared with the number of PMF CD34+ cells treated with cytokines alone or primary PMF CD34+ cells due to the effect of CMAs on inducing PMF CD34+ cell apoptosis. Surprisingly, the percentage of JAK2V617F/JAK2total in hCD45+ cells of mice transplanted with PMF CD34+ cells treated with cytokines alone also appeared to be decreased compared with that observed in hCD45+ BM cells of mice transplanted with primary PMF CD34+ cells, albeit to a lesser extent than that after CMA treatment. This observation is likely a consequence of the loss of the proliferative advantage of JAK2V617F-positive HSCs/HPCs due to the addition of cytokines to in vitro cultures which have been reported by several groups.16,28 Since the ability of primitive human hematopoietic cells to engraft sublethally irradiated immunodeficient mice is the standard surrogate in vivo assay for human HSCs, our findings suggest that in PMF both short-term HSCs and long-term HSCs are involved by the malignant process and can be eliminated by in vitro treatment with CMAs.
Sequential treatment with 5azaD/SAHA at concentrations that resulted in the elimination of JAK2V617F+ HPCs and SRCs was associated with increased apoptosis of PMF CD34+ cells but not normal CD34+ cells. Our laboratory has previously reported that the sequential treatment of normal HSCs with 5azaD and TSA results in their ability to undergo symmetrical cell division with retention of their marrow-repopulating potential.29-31 This behavior is in contrast to stem cells exposed in vitro to cytokines alone which undergo progressive loss of their proliferative and self-renewal capacity. Sequential treatment of normal stem cells with 5azaD/TSA results in the up-regulation of expression of number of genes previously implicated in HSCs self-renewal.31 The effects of CMAs on MPN CD34+ cells could, therefore, be due not only to their ability to inducing apoptosis and terminal differentiation of malignant stem cells, but also their ability to promote preferential expansion of residual pool of JAK2 wild-type stem cells. Such wild-type PMF stem cells are not necessary normal since they could contain additional genetic abnormalities (eg, TET2 or the additional sex combs like gene and the gene for isocitrate dehydrogenase 1).
We also showed that the sequential treatment of PMF CD34+ cells with 5azaD/SAHA resulted in the restoration of their capacity to migrate toward CXCL12 which was associated with the up-regulation of CXCR4 expression. Moreover, migratory 5azaD/SAHA treated PMF CD34+ cell contain greater number of JAK2V617F wild-type CD34+ cells. These findings suggest that the correction of PMF CD34+ cell migration after the treatment of CMAs could therefore be due either to the increased expression of CXCR4 by normal CD34+ cells or an increased number of normal HSCs/HPCs surviving CMA treatment relative to the number of malignant HSCs/HPCs, which has previously been reported by our laboratory to improve SRC homing.
In conclusion, our data clearly show that JAK2V617F+ SRCs and HPCs can be preferentially eliminated by sequential treatment with CMAs. Both SAHA and 2 DNA methyltransferase inhibitors are already approved to treat other hematologic malignancies providing a path for their evaluation in this patient population.20,32,33 CMA therapy thus represents a promising treatment option for PMF patients, which requires careful evaluation in well constructed clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was support by grants from the Myeloproliferative Disorders Foundation (to R.H.) and the National Cancer Institute (1P01CA108671 to R.H.).
National Institutes of Health
Authorship
Contribution: X.W. designed and performed the experiments, analyzed the data, and wrote the paper; W.Z., M.L., and Y. L. performed some of the experiments; J.T. performed FISH; M.X. reviewed the paper; V.N. analyzed FISH data; and R.H. designed the experiments, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, Division of Hematology/Oncology, Department of Medicine, Tisch Cancer Institute, Mount Sinai School of Medicine; 1 Gustave L. Levy Pl, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.