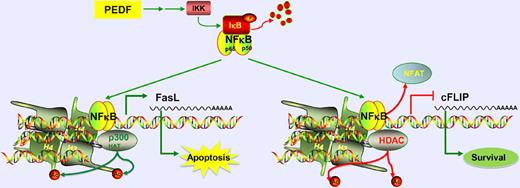
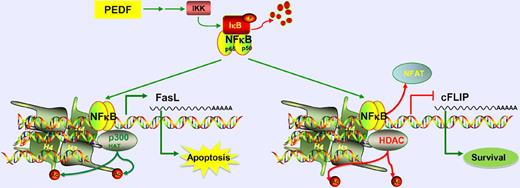
In this issue of Blood, Aurora and colleagues identify a novel mechanism for the inhibition of angiogenesis by PEDF and TSP1, mediated via chromatin remodeling and transcriptional regulation by NF-κB.1
Angiogenesis, formation of new vessels from preexisting ones, occurs both in physiologic and pathologic settings including tumor growth. There is a yet unproven hypothesis that in the physiologic nonangiogenic state, an equilibrium between endogenous antiangiogenic and proangiogenic molecules maintains the “angiogenic balance,” while in conditions such as cancer, an increased secretion of proangiogenic molecules shifts this balance to favor an angiogenic phenotype.2,3 While our knowledge of how proangiogenic molecules function has increased in the past few years, the mechanism of action associated with endogenous angiogenesis inhibitors is still largely unknown. Aurora et al address this unanswered question in this report.1
The endogenous angiogenesis inhibitor, PEDF, acts via NF-κB signaling to induce apoptosis of endothelial cells. NF-κB upregulates proapoptotic FasL expression by recruiting p300 HAT, causing histone acetylation. It also down-regulates prosurvival cFLIP by displacing NFAT and recruiting HDAC, causing histone deacetylation.
The endogenous angiogenesis inhibitor, PEDF, acts via NF-κB signaling to induce apoptosis of endothelial cells. NF-κB upregulates proapoptotic FasL expression by recruiting p300 HAT, causing histone acetylation. It also down-regulates prosurvival cFLIP by displacing NFAT and recruiting HDAC, causing histone deacetylation.
NF-κB is a transcription factor that can participate in both activation and repression of transcription. This is achieved in part by its interactions with histone deacetylases (HDACs) or histone acetyl transferases (HATs),4 which are major mediators of chromatin remodeling. NF-κB plays a dual role in cancer. It is speculated to have dose-dependent apoptotic or proliferative effects on cancer cells. Additionally, it can promote angiogenesis through induction of survival of endothelial cells and secretion of angiogenic factors by cancer cells.4,5 However, emerging evidence indicates that NF-κB signaling in endothelial cells induces apoptosis, leading into inhibition of angiogenesis.6 In this study, Aurora et al show that endogenous antiangiogenic molecules pigment-epithelium–derived factor (PEDF) and thrombospondin-1 (TSP1) activate NF-κB, through the canonical pathway via phosphorylation and degradation of NF-κB inhibitor IκB, eventually leading into apoptosis of endothelial cells. It is also identified that TSP1 and PEDF regulate NF-κB to reduce the ability of endothelial progenitors to assume an endothelial morphology and serve to influence the angiogenic phenotype. These provocative observations should be pursued further in the future. The evidence for the involvement of NF-κB in the action of PEDF is strong,7 whereas TSP1 is thought to also act via integrins, integrin-associated protein, or other matrix remodeling molecules.8 While this study provides definitive in vitro and in vivo evidence that NF-κB signaling is required for the apoptotic effect of PEDF on endothelial cells, such stringent assessment remains to be performed for TSP1 and other endogenous angiogenesis inhibitors. Such studies will offer crucial insight into a possible common mechanism of action for all endogenous angiogenesis inhibitors.
As a consequence of its release from IκB, NF-κB localization to nucleus increases and it is recruited to its target promoters including those of FasL and cFLIP. While FasL is a proapoptotic molecule, cFLIP is a survival factor. Interestingly, Aurora et al demonstrate that NF-κB acts as an activator of transcription of FasL, but functions as a repressor of cFLIP promoter. At the levels of FasL promoter, NF-κB acts to recruit p300 HAT, which is associated with an increase in acetylated histone H3. On the other hand, on the cFLIP promoter, it likely competes with the binding of NFATc2 transcription factor and displaces it. As NFATc2 drives expression of cFLIP, this results in the inhibition of cFLIP transcription. This group previously identified that PEDF reduces cFLIP expression via inhibition of NFATc2.9 Here, they provide novel evidence that indicates NF-κB is required in this pathway to displace NFATc2. Furthermore, NF-κB recruits HDAC1 to the cFLIP promoter and reduces the recruitment of p300 HAT, leading to a decrease in histone H3 and H4 acetylation. The basis of this dual action of NF-κB and how it specifically induces opposite effects at 2 different promoters needs further investigation. The authors observe that inhibition of NF-κB signaling in endothelial cells treated with vascular endothelial growth factor alone, in the absence of any antiangiogenic molecule treatment, also reduces cFLIP levels, suggesting that NF-κB up-regulates the transcription of cFLIP in the absence of any antiangiogenic signal. While more work needs to be done, this initial identification of a dual role for NF-κB in the context of proangiogenesis and antiangiogenesis is a striking finding.
Aurora et al also provide a therapeutic perspective in their report. Upon identification that NF-κB induced by PEDF regulates HATs and HDACs, they next assess whether HDAC inhibitor drugs such as SAHA or valproic acid can in combination with PEDF induce synergistic antiangiogenesis effect. This is particularly intriguing because HDAC inhibitors were previously shown to induce inhibition of angiogenesis.10-12 The present findings of Aurora et al seem to partially argue against these previous reports. The authors demonstrate here that HDAC activity is important for PEDF-mediated inhibition of angiogenesis via repression of cFLIP. To the contrary, HDAC inhibition mediated via histone acetylation of the FasL promoter would be expected to augment PEDF's effect. The authors attempt to address this contradiction by identifying that HDAC inhibitors have dose-dependent effects on angiogenesis when used in combination with PEDF. At high doses, they cause an up-regulation of cFLIP, which leads to a proangiogenic effect, whereas at low doses they enhance PEDF's effect leading to an antiangiogenic effect. Although the molecular basis for this observation was not elucidated, the authors do identify a potential role for HDAC inhibitors in regulating NF-κB acetylation, which, in turn, is thought to be important for its antiangiogenesis activity.
Collectively, in this study, Aurora et al provide novel insights regarding the role of NF-κB–mediated angiogenesis inhibition by PEDF and TSP1. This study offers a novel insight into the role of NF-κB and chromatin remodeling in angiogenesis regulation and how its dual role as a transcriptional activator and repressor may maintain the angiogenesis balance in physiologic states. Further elaboration on these observations will lead to a better design of combination therapeutic strategies to control angiogenesis in various pathologic states.
Conflict-of-interest disclosure: R.K. is a stock equity holder in EAI Corporation. The remaining author declares no competing financial interests. ■
REFERENCES
National Institutes of Health