Abstract
Extracellular factors control the angiogenic switch in endothelial cells (ECs) via competing survival and apoptotic pathways. Previously, we showed that proangiogenic and antiangiogenic factors target the same signaling molecules, which thereby become pivots of angiogenic balance. Here we show that in remodeling endothelium (ECs and EC precursors) natural angiogenic inhibitors enhance nuclear factor-κB (NF-κB) DNA binding, which is critical for antiangiogenesis, and that blocking the NF-κB pathway abolishes multiple antiangiogenic events in vitro and in vivo. NF-κB induction by antiangiogenic molecules has a dual effect on transcription. NF-κB acts as an activator of proapoptotic FasL and as a repressor of prosurvival cFLIP. On the FasL promoter, NF-κB increases the recruitment of HAT p300 and acetylated histones H3 and H4. Conversely, on cFLIP promoter, NF-κB increases histone deacetylase 1 (HDAC1), decreases p300 and histone acetylation, and reduces the recruitment of NFAT, a transcription factor critical for cFLIP expression. Finally, we found a biphasic effect, when HDAC inhibitors (HDACi) were used to test the dependence of pigment epithelial-derived factor activity on histone acetylation. The cooperative effect seen at low doses switches to antagonistic as the concentrations increase. Our study defines an interactive transcriptional network underlying angiogenic balance and points to HDACi as tools to manipulate the angiogenic switch.
Introduction
Angiogenesis, capillary formation from the preexisting vasculature, is crucial for tumor growth.1 Therapies targeting vascular endothelial growth factor (VEGF), platelet-derived growth factor, or their receptors2,3 underscore the impact of antiangiogenics but also highlight the ability of vessels to circumvent the blockade of a single angiogenic stimulus.4,5 Natural angiogenic inhibitors, which are often regarded as endothelial-specific tumor suppressors,6 are attractive in view of their ability to counteract multiple angiogenic stimuli and because of their specificity for remodeling endothelium.7 In activated endothelial cells (ECs), the inhibitors cause apoptosis by targeting molecules deployed by angiogenic stimuli.8 We recently documented the first of such events, in which proangiogenic and antiangiogenic factors cross-regulate an endothelial transcription factor (TF), nuclear factor of the activated T cells (NFAT), to turn the angiogenic switch on and off.9
We used promoter arrays to measure the activity of multiple TFs in remodeling ECs after exposure to the angioinhibitory protein pigment epithelial-derived factor (PEDF). We chose nuclear factor-κB (NF-κB) because it is implicated in angiogenesis,10 drives Fas ligand (FasL) expression,11 and is activated by PEDF in non-ECs.12 NF-κB can play 2 opposing roles in tumorigenesis. It induces immune cells to express inflammatory cytokines, which then enhance tumor cell survival13,14 and angiogenesis.15 On the other hand, constitutive NF-κB activation in tumor cells promotes apoptosis16 via death receptors and Fas/CD95 and thereby delays tumor progression.17-19
NF-κB can facilitate transcriptional activation or repression, depending on the cofactors available at the given promoter.20,21 It frequently interacts with histone-modifying enzymes to regulate gene expression that determines apoptosis versus survival.22 For example, in cooperation with histone deacetylases (HDACs), NF-κB blocks the expression of BNIP3,23 whereas the NF-κB/p300 histone acetyl transferase (HAT) complexes promote interleukin-8 expression.24
HDAC inhibitors (HDACi) hold significant promise as cancer therapeutics because of their ability to derepress tumor suppressors and proapoptotic genes, such as p53, VHL, and Fas,25 and to block proteins required for survival and metastases, including cFLIP.25 HDACi directly interfere with the growth and invasion of the tumor cells and create a microenvironment nonpermissive for tumor growth by disrupting angiogenesis.26,27 The application of HDACi in vivo for human use is hampered by the extremely high doses required to achieve efficacy, which cause nonspecific toxicity, thus precluding their use for long-term treatment.28
In this study, we demonstrate a novel role for NF-κB in determining EC fate by regulating transcriptional events that control apoptosis. NF-κB simultaneously acts as a transcriptional repressor for survival genes and as an activator for the apoptotic ones. Its dual activity is determined by promoter-specific chromatin remodeling mediated by the recruitment of HDACs or HAT p300, respectively. In addition, the reduced recruitment of NFAT at the cFLIP promoter on NF-κB engagement suggests that NF-κB may restrict cFLIP promoter binding by NFAT. Given the involvement of histone modifications, we investigated whether HDACi alter PEDF angioinhibitory activity and showed that vorinostat (suberoylanilide hydroxamic acid [SAHA]) and valproic acid (VA) cooperatively enhance PEDF antiangiogenic effects at low doses but antagonize PEDF activity at high doses. Our study defines the role for the NF-κB–NFAT duo in keeping angiogenic balance and offers the possibility to lower therapeutic doses of HDACi using them in combination with the antiangiogenic derivatives of PEDF or TSP1.
Methods
Cells and reagents
Information on cells and reagents is given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Promoter array analysis
Protein/DNA arrays (Panomics) for 56 transcription factors were used as recommended by the manufacturer and analyzed using ImageJ software (National Institutes of Health).
Immunofluorescence
Cells plated on coverslips were fixed in methanol/acetone (1:1), blocked (1 hour at room temperature in 4% donkey serum), and incubated overnight (4°C) with p65 antibodies and Cy3-conjugated secondary antibodies (supplemental Table 1). Images were obtained by fluorescent microscopy and quantified with MetaMorph software (Molecular Devices).
Immunoprecipitation and Western blotting
ECs were fractionated as described29 ; whole cell extracts were prepared with Boehringer buffer. Immunoprecipitation and Western blot analysis were performed on cell lysates using standard techniques. To measure phospho-IκBα, ECs were pretreated with proteasome inhibitor MG132 (Calbiochem) to preclude rapid protein turnover. Each Western blot was repeated a minimum of 3 times, and representative examples are shown. Densitometry was performed with ImageJ software.
ChIP
We used the EZ-ChIP kit (Upstate) following the manufacturer's protocol. Antibodies to precipitate DNA complexes are listed in supplemental Table 1. Primers for the promoter sequences (supplemental Table 2) were used for conventional or real-time polymerase chain reaction (PCR). All chromatin immunoprecipitation assay (ChIP) analyses were repeated at least 3 times, and all PCR reactions were performed at least twice. P values were determined by 1-way analysis of variance.
Conventional and quantitative PCR
Total RNAs were extracted and reverse transcribed with SuperScript kit (Promega). Quantitative PCR was performed with iQ SYBRGreen Supermix (Bio-Rad) in an MJ Research Chromo4 thermal cycler (primers, supplemental Table 2). Each experiment was repeated a minimum of 3 times and each sample amplified in triplicate. The results of 1 representative experiment are shown.
Apoptosis assay
Human microvascular endothelial cells (HMVECs) were plated on gelatinized coverslips (5 × 104 cells per well) in 24-well plates and fixed in 1% buffered paraformaldehyde. Apoptosis was detected using TdT-mediated dUTP nick end labeling (TUNEL assay; ApopTag kit, Chemicon). Images were obtained by fluorescent microscopy, quantified with MetaMorph software, and percentages of TUNEL-positive cells calculated. We evaluated 500 to 1000 cells (3-9 random fields) for each experimental condition. Normalized data from 3 independent experiments are presented.
Animals
Six- to 8-week-old female athymic nude mice (nu/nu) or C57BLJ6 mice (Harlan) were kept in pathogen-free environment; autoclaved water and γ-irradiated commercial diet were provided ad libitum. All manipulations were performed under sterile conditions following the institutional guidelines and in compliance with institutional guidelines for care and use of laboratory animals. The animal protocol was approved by the Northwestern University Animal Care and Use Committee.
In vivo angiogenesis assays
Corneal Micro-pocket Assay was performed as described previously30 : Hydron/Sucralfate (aluminum sucrose sulfate; Sigma-Aldrich) pellets contained PEDF, basic fibroblast growth factor (bFGF), and BMS-345541 at 50 ng, 25 ng, and 5μM/pellet, respectively. Pellets were implanted in a mouse cornea 0.5 to 1 mm from the vascular limbus. Angiogenesis was assessed by slit-lamp microscopy 5 days after implantation. P values were determined using Fisher Exact test to compare treatment groups. Matrigel Plug Assay was performed as in Quesada et al31 : PEDF, 34-mer, and BMS-345541 were incorporated in Matrigel at 10nM, 100nM, 5μM, and 2 to 200nM, respectively. Matrigel aliquots (500 μL/mouse) containing indicated substances were injected subcutaneously in the median abdominal area of anesthetized nude mice. Vorinostat and VA were administered intraperitoneally. Alternatively, HMVECs were infected with control adenovirus encoding β-Gal (AdC) or with adenoviral construct expressing IκB super-repressor (AdSR) and incorporated in Matrigel (105 cells/mL). After 10 to 13 days, the plugs were extracted, snap-frozen, and analyzed by immunostaining or in situ TUNEL. Quantitative analysis was performed using MetaMorph software. Directed In vivo Angiogenesis Assay (DIVAA; Trevigen) was carried out per the manufacturer's instructions. Angioreactors were filled with Matrigel with VEGF/bFGF cocktail with/without PEDF, 34-mer and BMS-345541 and implanted subcutaneously onto dorsal flanks of the nude mice. Vorinostat and VA were administered subcutaneously. The reactors were harvested 11 days after implantation and ECs quantified by fluorescein isothiocyanate-lectin staining followed by a bioluminescence measurement (480 nm excitation and 510 nm emission). For both the Matrigel and DIVAA, statistical significance was determined using 1-way analysis of variance.
Adenoviral infection
Replication-deficient type 5 adenovirus-expressing NF-κB super-repressor, a phosphorylation-deficient IκB variant (AdSR) under the control of cytomegalovirus promoter and control adenovirus expressing β-Gal (AdC) were provided by Dr D. Klumpp (Urology Department, Northwestern University) and propagated in HEK293 cells. Endothelial cells were infected at multiplicity of infection 100:1 and used for further analysis.
Results
PEDF and TSP1 stimulate NF-κB in the remodeling endothelium
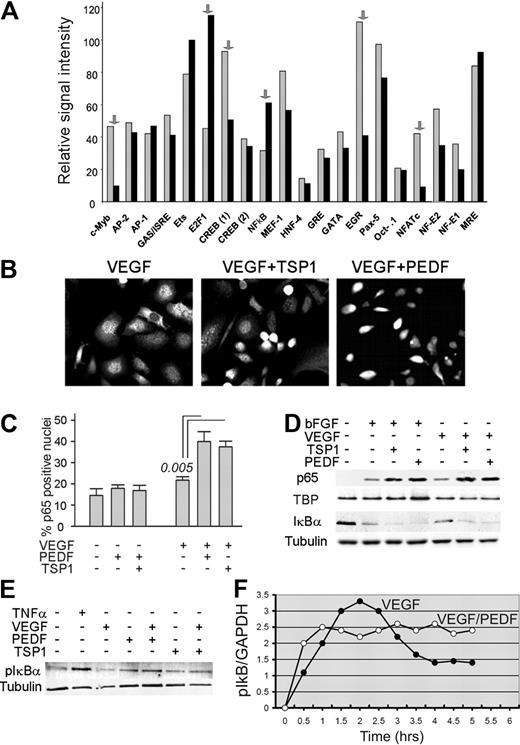
To identify TFs cotargeted by proangiogenic and antiangiogenic proteins, we used promoter array analysis. The comparison between HMVECs stimulated with VEGF alone and in the presence of PEDF (Figure 1A) showed that 6 factors, including NFATc2, were altered more than 2-fold. DNA binding was decreased for NFAT, c-Myb, CREB, and Ets-1 and increased for NF-κB and E2F1. We chose NF-κB because of its known role in PEDF function12 and in neovascular responses.32 In situ immunofluorescence (Figure 1B-C) and Western blotting analyses (Figure 1D) showed that both PEDF and TSP1 enhanced nuclear localization of NF-κB p65. In agreement with previous publications,32 VEGF and bFGF moderately increased p65 nuclear localization (Figure 1D) and caused a modest increase in phosphorylation and a decrease of the total levels of NF-κB inhibitor IκBα (Figure 1D-E). Both PEDF and TSP1 augmented IκBα phosphorylation and degradation (Figure 1D-E), suggesting that PEDF activates NF-κB via the canonical pathway.33 Electrophoretic mobility shift assay confirmed the increased DNA binding of NF-κB p65 and p50 caused by TSP1 and PEDF treatment (supplemental Figure 1A-B). Time-course studies of NF-κB activation showed that both PEDF and VEGF activated endothelial NF-κB in an oscillatory manner. In the presence of PEDF, the initial peak of activity occurred at 0.5 to 1.0 hours; the activation continued in an oscillatory manner with lower amplitude and an oscillation period of approximately 1.5 hours. In the presence of PEDF, the overall NF-κB activity remained high for at least 6 hours. In contrast, similar to the previously published observations,32 NF-κB induction by VEGF reached its peak at approximately 2 hours, trailed off by 4 hours, and remained low thereafter (Figure 1F).
Endothelial NF-κB is activated by angiogenic inhibitors. (A) Protein-DNA array data expressed as the relative signal intensity of transcription factor (TF) binding in human microvascular endothelial cells (HMVECs) activated with vascular endothelial growth factor (VEGF; 1 ng/mL, gray bars) and VEGF plus pigment epithelial-derived factor (PEDF; 20 nm, black bars): the arrows indicate transcription factors altered more than 2-fold. (B) Nuclear localization of NF-κB in HMVECs treated with VEGF (200 pg/mL), TSP1 (100nM), and PEDF (20nM) as indicated, fixed, and stained for p65. Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 40×/0.60 air objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (C) Quantitative analysis of the experiment in panel B. P value (determined by a 1-way analysis of variance) is shown where the difference with VEGF reached statistical significance. (D-E) Western blot analysis of NF-κB activation: nuclear extracts from HMVECs treated 30 minutes with indicated combinations of bFGF (10 ng/mL), VEGF (200 pg/mL), TSP1 (100nM), and PEDF (10nM) were probed for NF-κB p65; cytoplasmic extracts were probed for IκBα (D) or phospho-IκBα (E). To assess loading, the membranes were reprobed for TATA-binding protein (TBP) and tubulin. Tumor necrosis factor-α was used as a positive control. Three independent experiments were performed with similar results. (F) Time course of NF-κB activation was determined by measurements of IκB phosphorylation in ECs pretreated with proteasome inhibitor MG132 (30 minutes, 10μM). To quantify the results of the Western blot, we measured the phospho-IκB to GAPDH ratio and plotted it as a function of time on a linear scale. A representative of 3 independent experiments is shown.
Endothelial NF-κB is activated by angiogenic inhibitors. (A) Protein-DNA array data expressed as the relative signal intensity of transcription factor (TF) binding in human microvascular endothelial cells (HMVECs) activated with vascular endothelial growth factor (VEGF; 1 ng/mL, gray bars) and VEGF plus pigment epithelial-derived factor (PEDF; 20 nm, black bars): the arrows indicate transcription factors altered more than 2-fold. (B) Nuclear localization of NF-κB in HMVECs treated with VEGF (200 pg/mL), TSP1 (100nM), and PEDF (20nM) as indicated, fixed, and stained for p65. Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 40×/0.60 air objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (C) Quantitative analysis of the experiment in panel B. P value (determined by a 1-way analysis of variance) is shown where the difference with VEGF reached statistical significance. (D-E) Western blot analysis of NF-κB activation: nuclear extracts from HMVECs treated 30 minutes with indicated combinations of bFGF (10 ng/mL), VEGF (200 pg/mL), TSP1 (100nM), and PEDF (10nM) were probed for NF-κB p65; cytoplasmic extracts were probed for IκBα (D) or phospho-IκBα (E). To assess loading, the membranes were reprobed for TATA-binding protein (TBP) and tubulin. Tumor necrosis factor-α was used as a positive control. Three independent experiments were performed with similar results. (F) Time course of NF-κB activation was determined by measurements of IκB phosphorylation in ECs pretreated with proteasome inhibitor MG132 (30 minutes, 10μM). To quantify the results of the Western blot, we measured the phospho-IκB to GAPDH ratio and plotted it as a function of time on a linear scale. A representative of 3 independent experiments is shown.
TSP1 and PEDF induced NF-κB activation in EPCs
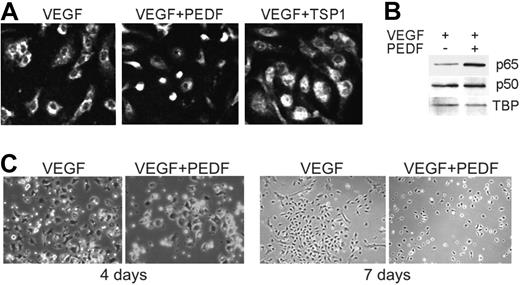
TSP1 has been previously shown to block endothelial progenitor cell (EPC) recruitment to the neovasculature.34 We analyzed NF-κB nuclear localization ex vivo, in VEGF-stimulated BM-derived EPCs35 cultured with VEGF in the absence or in the presence of TSP1 or PEDF. Both TSP1 and PEDF increased NF-κB nuclear localization (Figure 2A-B). Interestingly, the ability of EPCs to assume endothelial morphology and to form colonies in response to VEGF was also diminished by PEDF, suggesting that it can reduce EPC potential for vascular recruitment and differentiation (Figure 2C).
Angiogenic inhibitors activate NF-κB in endothelial precursor cells. (A-B) NF-κB nuclear localization in bone marrow endothelial progenitor cells (EPCs) stimulated to differentiate for 7 days with VEGF (10 ng/mL) and treated with TSP1 (100nM) or PEDF (10nM) was visualized by immunostaining for p65 (A) and measured by Western blot analysis of nuclear extracts for p65 (top) and p50 (middle; B). Loading was assessed by TBP (bottom). Three independent experiments were performed with similar results. (C) EPC colony formation with and without PEDF (10nM). Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 40×/0.60 air objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used.
Angiogenic inhibitors activate NF-κB in endothelial precursor cells. (A-B) NF-κB nuclear localization in bone marrow endothelial progenitor cells (EPCs) stimulated to differentiate for 7 days with VEGF (10 ng/mL) and treated with TSP1 (100nM) or PEDF (10nM) was visualized by immunostaining for p65 (A) and measured by Western blot analysis of nuclear extracts for p65 (top) and p50 (middle; B). Loading was assessed by TBP (bottom). Three independent experiments were performed with similar results. (C) EPC colony formation with and without PEDF (10nM). Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 40×/0.60 air objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used.
NF-κB is critical for PEDF antiangiogenic function
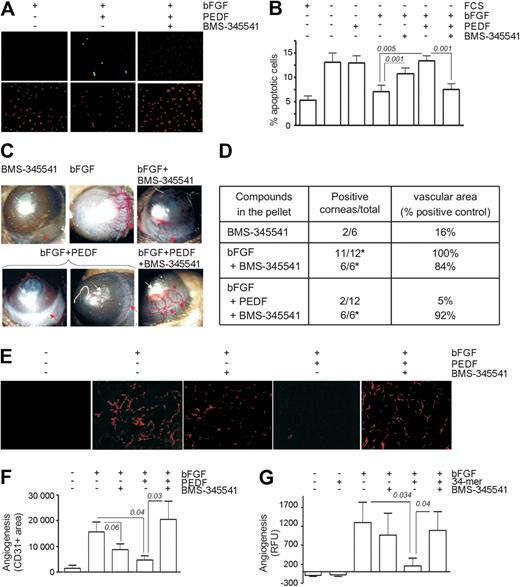
Multiple angiogenesis inhibitors, including PEDF, cause apoptosis in activated ECs.7 To investigate the role of NF-κB in PEDF-dependent apoptosis, we used BMS-345541, a specific in vitro and in vivo inhibitor of IκB kinase (IKK)α/β, the upstream activating kinases in the NF-κB pathway.36 In the presence of bFGF, HMVECs were protected from apoptosis caused by serum deprivation; but, when treated with PEDF, HMVECs underwent apoptosis despite the presence of bFGF (P = .04). This apoptosis was inhibited by 3μM BMS-345541, a dose that precludes NF-κB activation in multiple cell types36 (P = .002, Figure 3A-B).
Chemical inhibition of NF-κB abrogates PEDF antiangiogenic function. (A-B) Endothelial cell (EC) apoptosis shown in HMVECs treated with the indicated combinations of bFGF (10 ng/mL) and PEDF (10nM), with or without BMS-345541. (A) Representative images of apoptosis detected by in situ TUNEL (green), counterstained with propidium iodide (red). (B) Quantitative analysis of the data shown in panel A. Statistical significance was determined by a 1-way analysis of variance. (C) Representative images of mouse corneal angiogenesis induced by bFGF and blocked by PEDF, with or without BMS-345541. (D) Quantitative analysis of the experiment in panel C. Angiogenesis is scored as positive corneas of total implanted or as clockwork vascular area (percentage of bFGF control). P values (determined by Fisher Exact test) are shown where significant. (E-F) Angiogenesis was induced in Matrigel plugs with bFGF (250 ng/mL) and blocked by PEDF with or without BMS-345541. Plugs were sectioned and stained for CD31 (red). Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (F) Quantification of the experiment in panel E. P values are shown. (G) DIVAA assay: angioreactors containing Matrigel with indicated compounds were implanted in the flanks of nude mice for 13 days; ECs were visualized by exposure with fluorescein isothiocyanate-lectin and quantified by fluorimetry. P values (determined by analysis of variance) are shown.
Chemical inhibition of NF-κB abrogates PEDF antiangiogenic function. (A-B) Endothelial cell (EC) apoptosis shown in HMVECs treated with the indicated combinations of bFGF (10 ng/mL) and PEDF (10nM), with or without BMS-345541. (A) Representative images of apoptosis detected by in situ TUNEL (green), counterstained with propidium iodide (red). (B) Quantitative analysis of the data shown in panel A. Statistical significance was determined by a 1-way analysis of variance. (C) Representative images of mouse corneal angiogenesis induced by bFGF and blocked by PEDF, with or without BMS-345541. (D) Quantitative analysis of the experiment in panel C. Angiogenesis is scored as positive corneas of total implanted or as clockwork vascular area (percentage of bFGF control). P values (determined by Fisher Exact test) are shown where significant. (E-F) Angiogenesis was induced in Matrigel plugs with bFGF (250 ng/mL) and blocked by PEDF with or without BMS-345541. Plugs were sectioned and stained for CD31 (red). Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (F) Quantification of the experiment in panel E. P values are shown. (G) DIVAA assay: angioreactors containing Matrigel with indicated compounds were implanted in the flanks of nude mice for 13 days; ECs were visualized by exposure with fluorescein isothiocyanate-lectin and quantified by fluorimetry. P values (determined by analysis of variance) are shown.
To confirm the dependence of PEDF on NF-κB in vivo, we tested BMS-345541 in 3 angiogenesis models. In mouse cornea, NF-κB blockade with BMS-345541 abolished antiangiogenesis by PEDF (Figure 3C-D). Consistent with its effect on bFGF-induced EC survival, BMS-345541 showed a tendency to attenuate bFGF-induced angiogenesis (Figure 3D). However, the percentage of positive responses remained unaltered. In Matrigel plugs, PEDF significantly inhibited bFGF-induced angiogenesis (P = .046). Although BMS-345541 also showed a tendency to decrease bFGF-induced microvessel density, this trend did not reach statistical significance (P = .06). In contrast, BMS-345541 significantly diminished the PEDF antiangiogenic effect (P = .03, Figure 3E-F). In DIVAA, where angiogenesis is quantified as fluorescence of lectin-stained ECs, PEDF caused a statistically significant decrease in the number of ECs recovered from angioreactors (P = .034). This effect was also abolished by BMS-345541 treatment (P = .026, Figure 3G).
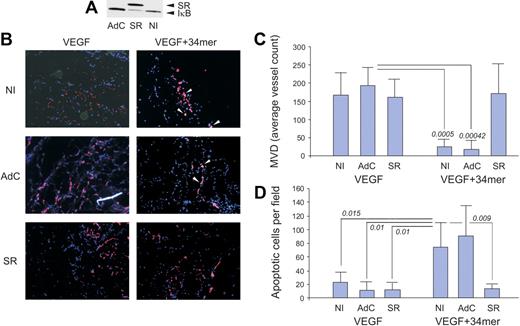
A more stringent method of NF-κB blockade, using an adenoviral vector expressing a phosphorylation defective IκB mutant termed IκB super-repressor (IκB-SR) yielded similar results. HMVECs incorporated in Matrigel plugs in nude mice actively participate in de novo angiogenesis.37 We infected HMVECs with either control adenovirus (AdC) or adenoviral vector expressing IκB-SR (AdSR). IκB-SR effectively displaced endogenous IκB as was confirmed by Western blot analysis (Figure 4A). HMVECs infected with either AdC or AdSR actively participated in VEGF-induced capillary formation (Figure 4B). The 34-mer potently inhibited VEGF-induced neovascularization by HMVECs infected with AdC; AdSR, like BMS-345541, strongly attenuated the 34-mer angioinhibitory activity (Figure 4B-C). Moreover, in vivo EC apoptosis caused by the 34-mer was also attenuated by AdSR but not by AdC (Figure 4B,D). These results indicate that NF-κB is pivotal for antiangiogenesis by PEDF.
The IκB super-repressor blocks PEDF-induced antiangiogenesis in vivo. HMVECs were infected with adenoviral vector expressing IκB super-repressor (SR) or with control adenoviral vector expressing β-Gal (AdC). Noninfected HMVECs (NI) were used as control. Total cell extracts were collected and analyzed by Western blot for IκB (A). Note decreased endogenous IκB levels on IκB-SR overexpression. (B) HMVECs, noninfected or infected with AdSR or AdC, were incorporated into subcutaneous Matrigel plugs implanted in nude mice. Angiogenesis was induced with VEGF (200 ng/mL) and blocked by 100nM of the PEDF 34-mer peptide (both incorporated in the Matrigel). At the completion of experiment, the plugs were stained for CD31 (red), apoptosis was visualized by in situ TUNEL (green), and the nuclei highlighted with 4,6-diamidino-2-phenylindole (blue). Apoptotic cells appear white because of superimposed blue, red, and green (white arrows). Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (C) Digital images were taken and microvessel density evaluated using MetaMorph software. P values are shown. (D) Apoptosis evaluated on digital images with MetaMorph software. P values were determined by analysis of variance.
The IκB super-repressor blocks PEDF-induced antiangiogenesis in vivo. HMVECs were infected with adenoviral vector expressing IκB super-repressor (SR) or with control adenoviral vector expressing β-Gal (AdC). Noninfected HMVECs (NI) were used as control. Total cell extracts were collected and analyzed by Western blot for IκB (A). Note decreased endogenous IκB levels on IκB-SR overexpression. (B) HMVECs, noninfected or infected with AdSR or AdC, were incorporated into subcutaneous Matrigel plugs implanted in nude mice. Angiogenesis was induced with VEGF (200 ng/mL) and blocked by 100nM of the PEDF 34-mer peptide (both incorporated in the Matrigel). At the completion of experiment, the plugs were stained for CD31 (red), apoptosis was visualized by in situ TUNEL (green), and the nuclei highlighted with 4,6-diamidino-2-phenylindole (blue). Apoptotic cells appear white because of superimposed blue, red, and green (white arrows). Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (C) Digital images were taken and microvessel density evaluated using MetaMorph software. P values are shown. (D) Apoptosis evaluated on digital images with MetaMorph software. P values were determined by analysis of variance.
PEDF-induced NF-κB enhances FasL expression
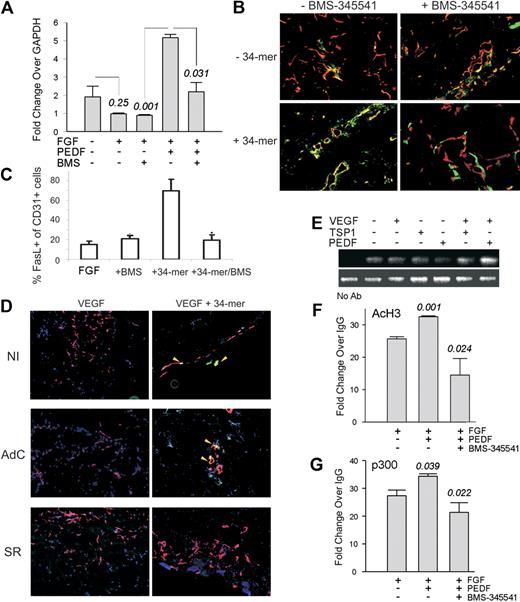
FasL/CD95L, a member of the tumor necrosis factor receptor superfamily, is critical for PEDF antiangiogenic activity.8 NF-κB can drive FasL expression in non-ECs.38 We therefore investigated whether NF-κB could contribute to FasL induction by PEDF and TSP1. PEDF caused a 2- to 3-fold increase in FasL mRNA, which was inhibited by BMS-345541 (Figure 5A). In Matrigel plugs, PEDF antiangiogenic peptide (the 34-mer) increased FasL presentation on the microvascular ECs (Figure 5B-C). This increase was visibly diminished in the presence of BMS-345541 (Figure 5B-C) and on infection by AdSR (but not by AdC; Figure 5D). Neither BMS-345541 nor AdSR had significant effect on FasL presentation in the presence of bFGF or VEGF alone (Figure 5B-D). The expression of cognate Fas receptor remained unaffected by the 34-mer and insensitive to the NF-κB blockade (supplemental Figure 2). ChIP with the antibodies for p65, acetylated histone H3, and p300 HAT, followed by quantitative PCR with primers flanking the NF-κB binding site on the FasL promoter showed that TSP1 and PEDF increased NF-κB recruitment to the FasL promoter (Figure 5F). The increased NF-κB expression was associated with chromatin modifications consistent with transcriptional activation, that is, an increase in acetylated histone H3 (acH3) and of the HAT, p300. Both changes were attenuated in the presence of BMS-345541 (Figure 5F-G). These data imply a critical role for NF-κB in the transcriptional activation of FasL/CD95L by PEDF.
PEDF increases FasL expression by activating chromatin at the FasL promoter. (A) Quantitative PCR showing a 6-fold increase in FasL mRNA in ECs treated with bFGF plus PEDF (P < .001) reversed by BMS-345541 (P < .031). (B) Mice were injected with Matrigel plugs containing bFGF, 34-mer peptide, and BMS-345541 where indicated. Sectioned plugs were stained for CD31 (red) and FasL (green). Colocalization of red and green fluorescence appears yellow. (C) Digital images (2 or 3 sections per treatment condition, 3 or 4 fields per section) were quantified using MetaMorph software. The percentages of the FasL-positive ECs (yellow) of the total ECs (red and yellow) were calculated. *Significantly different value from bFGF alone (P < .001). (D) Mice were injected with Matrigel containing control, noninfected HMVECs (NI), or HMVECS infected with adenoviral IκB super-repressor (SR) or β-Gal (AdC). Angiogenesis was induced with VEGF (200 ng/mL) and blocked with 100nM PEDF 34-mer peptide; 5-μm sections were stained for CD31 (red) and FasL (green) as in panel B. Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (E) TSP1 and PEDF increased NF-κB recruitment to the FasL promoter: ChIP was performed with p65 antibody, followed by PCR with primers for the FasL promoter fragment encompassing the κB consensus sequence. (F-G) PEDF increased HAT recruitment and histone acetylation at the FasL promoter: ChIP with the antibodies for AcH3 (F) and p300 (G) followed by quantitative PCR for the same fragment of FasL promoter. Note significant increases in AcH3 (P < .01) and p300 (P = .04) in the presence of PEDF. Both are reversed by the IKK inhibitor, BMS-345541 (P < .025, in both cases).
PEDF increases FasL expression by activating chromatin at the FasL promoter. (A) Quantitative PCR showing a 6-fold increase in FasL mRNA in ECs treated with bFGF plus PEDF (P < .001) reversed by BMS-345541 (P < .031). (B) Mice were injected with Matrigel plugs containing bFGF, 34-mer peptide, and BMS-345541 where indicated. Sectioned plugs were stained for CD31 (red) and FasL (green). Colocalization of red and green fluorescence appears yellow. (C) Digital images (2 or 3 sections per treatment condition, 3 or 4 fields per section) were quantified using MetaMorph software. The percentages of the FasL-positive ECs (yellow) of the total ECs (red and yellow) were calculated. *Significantly different value from bFGF alone (P < .001). (D) Mice were injected with Matrigel containing control, noninfected HMVECs (NI), or HMVECS infected with adenoviral IκB super-repressor (SR) or β-Gal (AdC). Angiogenesis was induced with VEGF (200 ng/mL) and blocked with 100nM PEDF 34-mer peptide; 5-μm sections were stained for CD31 (red) and FasL (green) as in panel B. Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (E) TSP1 and PEDF increased NF-κB recruitment to the FasL promoter: ChIP was performed with p65 antibody, followed by PCR with primers for the FasL promoter fragment encompassing the κB consensus sequence. (F-G) PEDF increased HAT recruitment and histone acetylation at the FasL promoter: ChIP with the antibodies for AcH3 (F) and p300 (G) followed by quantitative PCR for the same fragment of FasL promoter. Note significant increases in AcH3 (P < .01) and p300 (P = .04) in the presence of PEDF. Both are reversed by the IKK inhibitor, BMS-345541 (P < .025, in both cases).
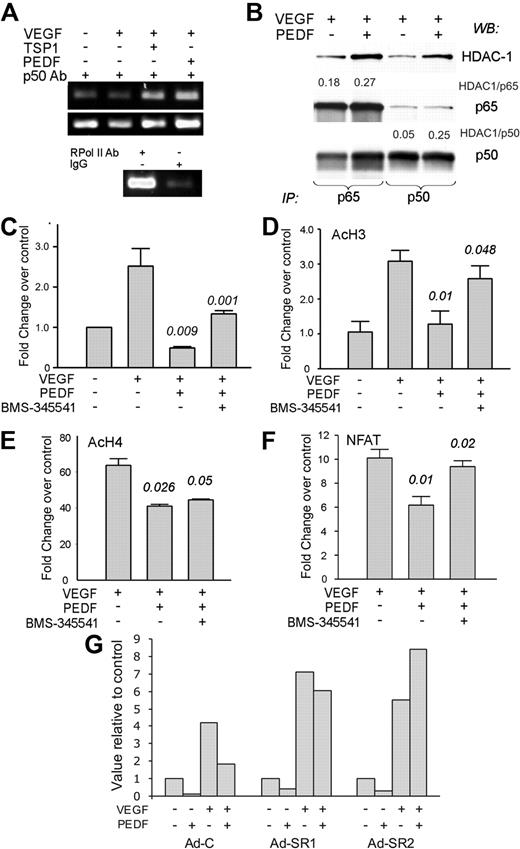
NF-κB restricts NFATc2 promoter binding and causes transcriptional repression of cFLIP
Inhibitors of angiogenesis sensitize remodeling ECs to apoptotic death by decreasing endogenous survival factors, including Survivin, XIAP, cFLIP, and Bcl-2.8,39 We have previously shown that PEDF suppresses cFLIP by blocking the activity of NFATc2 transcription factor.8 Because NF-κB drives cFLIP expression in other systems,40 we did not expect it to participate in cFLIP blockade by PEDF. As shown previously, PEDF decreased cFLIP levels in bFGF-stimulated cells (supplemental Figure 3B). Unexpectedly, ChIP with p50 antibodies followed by amplification of the κB-containing fragment of the cFLIP promoter showed increased p50 binding (Figure 6A), leading us to hypothesize that NF-κB might repress cFLIP transcription. Immunoprecipitation of the nuclear extracts with p65 and p50 antibodies showed a 4- to 5-fold increased HDAC1 association with p50 and a less pronounced 1.5-fold increase in HDAC1 association with p65, suggesting transcriptional repression rather than activation41 (Figure 6B; and data not shown). PEDF also decreased the recruitment of p300 and of acetylated histones AcH3 and AcH4 at the cFLIP promoter (Figure 6C-E). This decrease was partially reversed by BMS-345541 (Figure 6C-D), pointing to a role for NF-κB in cFLIP repression. As was shown previously, cFLIP protein was reduced in the presence of TSP1 or PEDF (supplemental Figure 3A): this decrease was not apparent in the presence of BMS-345541 (supplemental Figure 3B). However, treatment with BMS-345541 reduced cFLIP levels in the ECs stimulated with VEGF alone, suggesting a role for NF-κB in cFLIP expression on EC activation (supplemental Figure 3).
PEDF increases the binding of NF-κB, p300, and histone acetylation while restricting NFAT binding at cFLIP promoter. (A) ChIP analysis of NF-κB binding to the cFLIP promoter: DNA-protein complexes from HMVECS treated as indicated were precipitated with p50 antibody and amplified with primers for the area flanking the κB-binding site. Immunoprecipitation with antibody to RNA polymerase II and IgG served as positive and negative controls, respectively. (B) HMVECs were treated with VEGF plus or minus PEDF, nuclear extracts precipitated with the antibodies for p65 (left) or p50 (right), and analyzed by Western blot for HDAC1, p65 and p50, respectively. The blots were assessed by densitometry and the ratio between HDAC1 and p65 or p50 calculated (see numbers above the panels). A representative of 3 independent experiments is shown. (C) ChIP was performed with p300 antibodies, followed by quantitative PCR with the primers for the same cFLIP promoter fragment as in panel A. (D-E) ChIP was performed with the antibodies for AcH3 (D) and AcH4 (E), followed by quantitative PCR with the same primers. (F) ChIP was performed with the antibodies for NFATc2 and quantitative PCR with the same primer set. Statistical significance was calculated by 1-tailed Student t test. P values reflect the comparisons of VEGF with VEGF plus PEDF and VEGF plus PEDF with VEGF plus PEDF plus BMS, respectively. (G) ECs were infected with AdC or Ad-SR, treated for 4 hours as indicated (1 ng/mL VEGF, 20 ng/mL PEDF), and compared with the untreated control. The results of the 2 independent experiments are shown (Ad-SR1 and Ad-SR2, respectively).
PEDF increases the binding of NF-κB, p300, and histone acetylation while restricting NFAT binding at cFLIP promoter. (A) ChIP analysis of NF-κB binding to the cFLIP promoter: DNA-protein complexes from HMVECS treated as indicated were precipitated with p50 antibody and amplified with primers for the area flanking the κB-binding site. Immunoprecipitation with antibody to RNA polymerase II and IgG served as positive and negative controls, respectively. (B) HMVECs were treated with VEGF plus or minus PEDF, nuclear extracts precipitated with the antibodies for p65 (left) or p50 (right), and analyzed by Western blot for HDAC1, p65 and p50, respectively. The blots were assessed by densitometry and the ratio between HDAC1 and p65 or p50 calculated (see numbers above the panels). A representative of 3 independent experiments is shown. (C) ChIP was performed with p300 antibodies, followed by quantitative PCR with the primers for the same cFLIP promoter fragment as in panel A. (D-E) ChIP was performed with the antibodies for AcH3 (D) and AcH4 (E), followed by quantitative PCR with the same primers. (F) ChIP was performed with the antibodies for NFATc2 and quantitative PCR with the same primer set. Statistical significance was calculated by 1-tailed Student t test. P values reflect the comparisons of VEGF with VEGF plus PEDF and VEGF plus PEDF with VEGF plus PEDF plus BMS, respectively. (G) ECs were infected with AdC or Ad-SR, treated for 4 hours as indicated (1 ng/mL VEGF, 20 ng/mL PEDF), and compared with the untreated control. The results of the 2 independent experiments are shown (Ad-SR1 and Ad-SR2, respectively).
Our previous work showed that PEDF lowers cFLIP expression by opposing NFAT nuclear localization and expression of NFAT target genes.9 In addition, it has been speculated that NF-κB and NFAT may displace each other from similar DNA-binding sites.42 ChIP with NFATc2 antibodies and cFLIP promoter primers showed abundant NFAT binding to the cFLIP promoter in the presence of VEGF, which was restricted by PEDF treatment. However, when NF-κB activation was hampered in the presence of BMS-345541, PEDF inhibition of the NFAT binding was relieved (Figure 6F), suggesting that the binding was mutually exclusive. The infection with adenovirally expressed IκB-SR caused an even more dramatic effect. Similar to the BMS-345541 treatment, VEGF-stimulated ECs infected with AdC showed increased NFATc2 binding to the cFLIP promoter, which was relieved by PEDF. ECs infected with Ad-SR showed a tendency toward more effective NFATc2 binding, which was retained despite the presence of PEDF (Figure 6G).
HDACi interact with antiangiogenic factors in a biphasic fashion
The recruitment of HATs or HDACs is crucial to the subsequent histone modifications, which determine gene-specific regulation of NF-κB transcriptional activity in the presence of PEDF and TSP1. On the other hand, HDACi have been shown to suppress tumor angiogenesis in multiple systems.25 To solve this apparent paradox, we analyzed the effects of 2 HDACi on PEDF antiangiogenic activity. We used a potent HDACi, SAHA (or vorinostat), and a less potent one, VA. SAHA alone blocked baseline cFLIP expression in a dose-dependent manner (supplemental Figure 4A). In contrast, together with PEDF, SAHA at less than or equal to 30μM enhanced cFLIP repression; whereas at higher doses (100μM), it relieved cFLIP repression by PEDF (supplemental Figure 4A). Conversely, SAHA alone increased baseline FasL expression but had little or no effect in combination with PEDF (supplemental Figure 4B).
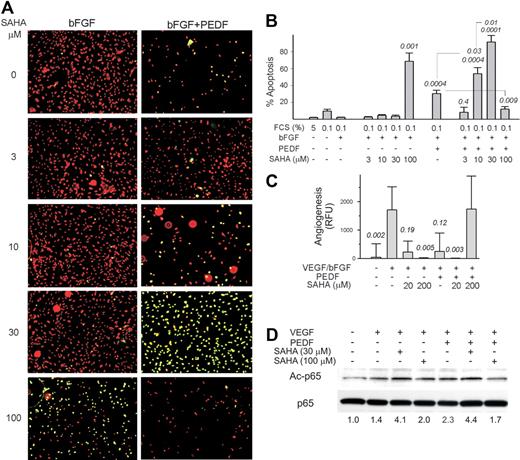
The combination of HDACi with PEDF had similar biphasic effects on EC apoptosis in vitro and angiogenesis in vivo. At lower doses, SAHA and VA enhanced apoptosis by PEDF. However, at more than or equal to 30μM SAHA and more than or equal to 100μM VA, PEDF-induced EC apoptosis was strongly attenuated (Figure 7A-B; supplemental Figure 5A). In DIVAA, PEDF reduced angiogenesis by 70% to 85% (Figure 7C). SAHA alone was antiangiogenic at 20 and 200μM. In contrast, 20μM SAHA enhanced PEDF action, whereas 200μM SAHA and PEDF showed a proangiogenic effect (Figure 7C). VA showed a similar tendency, whereas it was cooperative with PEDF at low doses and antagonistic at high doses (supplemental Figure 5B). Acetylation has been described to be crucial for NF-κB signaling. It was shown to alter the activity of NF-κB family members and their association with enhancing or repressing regulatory factors.43 When NF-κB acetylation was measured by Western blot, in the ECs activated with VEGF we observed a modest increase in acetylated NF-κB p65 in the presence of PEDF, which was further augmented by the low, proapoptotic concentrations of SAHA (30μM). In contrast, at a higher dose (100μM), SAHA opposed the increased NF-κB acetylation in PEDF-treated remodeling ECs (Figure 7D).
HDAC inhibitors have a biphasic effect on antiangiogenesis by PEDF. (A) HMVECs grown on coverslips in low serum were stimulated with bFGF and treated with PEDF (10nM) and/or HDAC inhibitor vorinostat (SAHA, 0-100μM as indicated) overnight for 16 hours; the cells were counterstained with propidium iodide (red) and apoptosis detected by TUNEL (green). Representative overlay images are shown. Apoptotic ECs appear yellow. Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (B) The experiment in panel A was quantified using MetaMorph software. Note the apoptotic effect of vorinostat alone at 100μM (P < .001), a dose-dependent enhancement of PEDF apoptotic activity by vorinostat at concentrations up to 30μM, and the inhibition of PEDF-dependent apoptosis by 100μM vorinostat. (C) DIVAA was performed with fixed concentrations of PEDF antiangiogenic peptide (the 34-mer, 100nM) and increasing doses of SAHA. Note similar cooperation between PEDF and 20μM SAHA (P < .005) and the reversal of PEDF angioinhibitory activity by SAHA at high dose (200μM, P = .03). (D) The acetylation of p65 was measured by Western blot with phospho-specific antibodies. Three independent experiments were performed with similar results. The ratio between phospho-p65 and total p65 is shown below.
HDAC inhibitors have a biphasic effect on antiangiogenesis by PEDF. (A) HMVECs grown on coverslips in low serum were stimulated with bFGF and treated with PEDF (10nM) and/or HDAC inhibitor vorinostat (SAHA, 0-100μM as indicated) overnight for 16 hours; the cells were counterstained with propidium iodide (red) and apoptosis detected by TUNEL (green). Representative overlay images are shown. Apoptotic ECs appear yellow. Images were viewed with a Nikon Eclipse TE 2000-U microscope with a 10×/0.45 objective and Fluoromount G imaging medium (Southern Biotech), and captured with a Nikon LH-M100 C-1 camera. Metamorph, Adobe Photoshop CS3 Extended Version 10.01, and Corel Photo-Paint 10 software were used. (B) The experiment in panel A was quantified using MetaMorph software. Note the apoptotic effect of vorinostat alone at 100μM (P < .001), a dose-dependent enhancement of PEDF apoptotic activity by vorinostat at concentrations up to 30μM, and the inhibition of PEDF-dependent apoptosis by 100μM vorinostat. (C) DIVAA was performed with fixed concentrations of PEDF antiangiogenic peptide (the 34-mer, 100nM) and increasing doses of SAHA. Note similar cooperation between PEDF and 20μM SAHA (P < .005) and the reversal of PEDF angioinhibitory activity by SAHA at high dose (200μM, P = .03). (D) The acetylation of p65 was measured by Western blot with phospho-specific antibodies. Three independent experiments were performed with similar results. The ratio between phospho-p65 and total p65 is shown below.
Discussion
Few studies offer analyses of transcriptional events in the endothelium during vascular remodeling or regression. Ets, GATA, and KLF superfamilies,44-46 NFAT,47 and NF-κB32 are identified as endothelial TFs critical for vascular remodeling, and FOXO, PPARγ, and p53 are crucial for vascular regression.48-51 Whereas GATA and KLF factors are strictly proangiogenic, Ets family members flip the angiogenic switch both ways.46
In contrast to the studies based on gene array analyses and thus overlooking posttranscriptional TF modulation, we used promoter arrays, which register TF-binding activity. We focused on NF-κB for its importance for angiogenesis, survival, and apoptosis, and its connection with PEDF.12 Whereas NF-κB contributes to EC survival in vitro and NF-κB blockade in vivo sensitizes ECs to stress-induced apoptosis, augmented tumor angiogenesis in animals expressing the IκB super-repressor suggests additional complexity.52 We have demonstrated the role of NF-κB in the endothelial-specific action of natural angiogenesis inhibitors. Moreover, in bone marrow-derived EPCs, PEDF increased NF-κB activity and decreased EC colonies, suggesting that NF-κB activation by PEDF may interfere with EPC differentiation and proliferation.
As expected, we observed NF-κB induction by angiogenic stimuli; however, the inhibitors further enhanced NF-κB nuclear translocation. Closer examination pointed to a role of NF-κB in EC apoptosis because BMS-345541, an inhibitor of upstream activating IKK kinase(s), rescued ECs from apoptosis by PEDF. In agreement with the existing computational model of activation,53 both angiogenic inhibitors and stimuli activated endothelial NF-κB in an oscillatory manner. However, in the presence of PEDF, the oscillation period of IκB phosphorylation was approximately 1.0 to 1.5 hours, which reflects intermediate feedback and low damping. After initial significant activation, the subsequent oscillations were relatively modest, whereas overall activity remained high for at least 6 hours. In contrast, similar to the previously published observations,32 IκB induction by VEGF reached its peak at approximately 2 hours and trailed off by 4 hours (a 4-hour period). Such kinetics reflects high feedback and high damping rates. For c-Jun N-terminal kinase, transient versus sustained activation results in survival versus apoptosis, respectively.54,55 Feasibly, distinct kinetics patterns of NF-κB activation similarly affect EC cell survival.
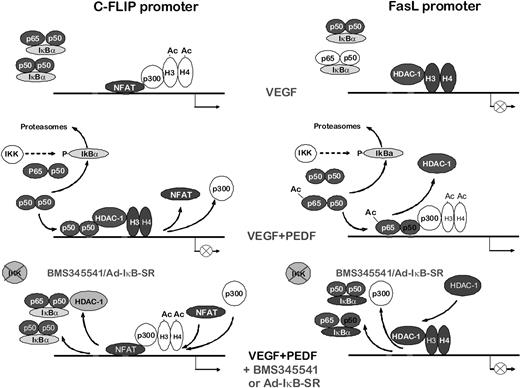
Our data delineate the mechanism by which NF-κB regulates angiogenesis (Figure 8). Previous studies indicate FasL and cFLIP as opposing forces deciding EC fate.9 Surprisingly, NF-κB regulated the transcription of both, but in opposite ways: it acts as a repressor for the antiapoptotic, angiogenic cFLIP and as an activator for the apoptotic, antiangiogenic FasL. In VEGF-stimulated endothelium, NFAT binds the cFLIP promoter and recruits p300, causing histone acetylation and gene activation. At the same time, the FasL promoter is rendered inactive, as indicated by the deacetylated state of histones H3 and H4, presumably by the class I HDACs. On PEDF treatment, NF-κB is released from the complexes with IκBα and translocated to the nuclei. At the cFLIP promoter, NF-κB (possibly p50 homodimers) displaces NFATc2 and recruits HDAC-1 causing deacetylation of histones and repression. At the FasL promoter, p65/p50 heterodimers are recruited with increased affinity because of the increased p65 acetylation. They increase p300 recruitment and histone acetylation causing higher FasL expression. When NF-κB activity is attenuated by BMS-345541 or the IκB-SR, the cFLIP and FasL promoters return to their “angiogenic” state (Figure 8). This pathway may be common for multiple angiogenic inhibitors because endostatin also blocks cFLIP expression and modulates FasL.56
Scheme of NF-κB–dependent antiangiogenic events. NF-κB–dependent transcriptional events in VEGF-stimulated ECs, inhibitor-treated ECs, and inhibitor-treated ECs after NF-κB blockade.
Scheme of NF-κB–dependent antiangiogenic events. NF-κB–dependent transcriptional events in VEGF-stimulated ECs, inhibitor-treated ECs, and inhibitor-treated ECs after NF-κB blockade.
The amount of NF-κB in the nucleus is sufficient to occupy all κB sites in the genome; however, target availability is restricted by promoter context, chromatin composition, coactivators, and/or corepressors available.57,58 NF-κB can act as transcriptional activator, but also as a repressor, specifically for prosurvival genes, such as IAPs.59 The increase in apoptosis by BMS-345541 alone could be the result of the repression of these factors or of other endothelial molecules, such as ICAM-1 or VCAM-1.60 We show that NF-κB simultaneously acts as an activator for the apoptotic and repressor for survival genes, a rare example of a single transcription factor performing opposing functions in the same cell population.
In addition to the recruitment of active and inactive chromatin to the promoter regions containing κB sites, NF-κB restricts the binding of NFATc2 to the cFLIP promoter. The structure of NFAT and NF-κB DNA-binding domains is strikingly similar; therefore, hypothetically, they could compete for the same promoter regions,61 although the displacement has never been shown directly. On cFLIP promoter, NF-κB and NFAT sites are located in close proximity (240 and 2000 bp apart). ChIP showed that the PEDF-induced increase in NF-κB binding and NFAT dissociation from the same region of the cFLIP promoter was reversed by BMS-345541 and by IκB super-repressor, an indirect demonstration of their competition for the DNA binding. The opposing effects on angiogenesis by NFAT and NF-κB are strikingly similar to those of Ets family members where Tel, Net, and Erf suppress pro-angiogenic transcriptional activity of Ets-1, Fli, and Egr.46,62
Angiogenesis inhibitors, such as Avastin, Lucentis, Sunitinib, and Sorafenib, become increasingly popular in the clinic.3,7 However, resistance to VEGF-directed agents can lead to tumor reliance on secondary cytokines, such as interleukin-8, to escape from angiogenic surveillance.63 Natural inhibitors and derivatives are less potent than single target agents. However, their efficacy against multiple stimuli presents an advantage; some (eg, TSP2 and endostatin) also restrict lympangiogenesis.64 The efficacy of antiangiogenics may be improved by combined use with chemotherapy or other antiangiogenics. Advantages of combined treatments have been demonstrated for Avastin, PIGF antibody, and ABT-510.27,31,65,66 Our study adds HDACi to the list of possible combinations. HDACi have been successfully used with Sutent and ABT-510, a TSP1 peptide mimic.27,65 In vivo, HDACi reach efficacy at high doses, causing nonspecific toxicity.28 In this study, we demonstrate cooperative antiangiogenic effects of PEDF and HDACi, when the latter are used at low rather than high doses, which may be the result of cooperative NF-κB acetylation by PEDF and low, but not high, doses of HDACi. Our data provide a compelling rationale for the use of low-dose HDACi with TSP1 or PEDF to combat aberrant angiogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (R01 HL68033 and R01HL077471, O.V.V.; T32 DK62716, A.B.A.) and the American Cancer Society (PF-06-256-01-CSM, A.B.A.; and R01 HL057516, D.L.).
National Institutes of Health
Authorship
Contribution: A.B.A. performed the main bulk of research, designed experiments, analyzed data, and composed the preliminary draft of the manuscript; D.B. designed and performed critical experiments and participated in data analysis and writing; Y.M. performed important experiments; T.A.Z. participated in the initial findings; C.S.-M. performed cloning and isolation of all viral constructs and particles; M.A.R. provided critical technical expertise; D.L. provided critical reagents; and O.V.V. supervised research, designed experiments, performed data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olga V. Volpert, Department of Urology, Northwestern University Feinberg School of Medicine, 303 E Chicago Ave, Chicago, IL 60611; e-mail: olgavolp@northwestern.edu.