Abstract
Overexpression of a constitutively active form of Stat5b (Stat5b-CA) increases regulatory T cells (Tregs). We show that Stat5b-CA transgenic (TG) CD4+ T cells had a markedly reduced graft-versus-host disease (GVHD) capacity versus wild-type (WT) T cells. Stat5b-CA TG versus WT CD4+ T cells had a higher proportion of Tregs, which were superior in suppressing alloresponses mediated by CD4+CD25− effector T cells (Teffs). By day 5 after transplantation, Stat5b-CA TG Tregs had expanded approximately 3-fold more than WT Tregs. Purified Stat5b-CA TG Tregs added to WT CD4+CD25− Teffs were superior on a per-cell basis for inhibiting GVHD versus WT Tregs. Surprisingly, rigorously Treg-depleted Stat5b-CA TG versus WT CD4+CD25− Teffs caused less GVHD lethality associated with diminished Teff proinflammatory and increased Th2 anti-inflammatory cytokine responses. Reduced GVHD by Stat5b-CA TG versus WT Teffs could not be explained by conversion into Tregs in day 10 posttransplantation spleen or small intestine. In addition, Stat5b-CA TG Teffs retained a graft-versus-leukemia response. These results indicate a major role for Stat5 in Treg expansion and potency along with a lesser but significant role in Teff activation and suggest a strategy of pharmacologic Stat5b up-regulation as a means of decreasing GVHD while retaining a graft-versus-leukemia effect.
Introduction
Cytokines regulate survival, proliferation, and differentiation of various immune cells, and those using the γc chain play a major role in lymphocyte homeostasis. Interleukin-2 (IL-2), IL-7, and IL-15 interactions with their receptors are essential for normal B- and T-cell development and peripheral T-cell homeostasis and proliferation.1-3 The major pathways activated on γc receptor activation are Jak/Stat, MAPK, and PI3K.4 Although these signals lead to changes in cell proliferation, differentiation, and survival in several T-cell lineages, the transcription factor Stat5 is particularly important for CD4+CD25+FoxP3+ regulatory T-cell (Treg) development and homeostasis in mice and humans.5-8 T-cell specific deficiency in both homologs, Stat5a and Stat5b, resulted in significantly decreased numbers of Tregs, as well as CD8+, CD4+, and γδ T-cell numbers in the thymus and in the periphery in mice.7,9 Conversely, transgenic (TG) expression of a constitutively active form of Stat5b (Stat5b-CA) in T and B cells resulted in an increase in Tregs, CD8+, CD4+, γδ, and natural killer T-cell numbers.5 Deletion or overexpression of Stat5 affected CD8+ T cells more than CD4+ T cells, with the exception of Tregs. CD8+ T cells in Stat5b-CA TG mice were increased far more than CD4+ T cells and exhibited a memory phenotype, whereas most CD4+ T cells maintained a naive phenotype. The majority of CD4+CD25+ T cells from Stat5b-CA TG mice expressed FoxP3 and suppressed wild-type (WT) effector T-cell (Teff) responses in vitro. On T-cell receptor (TCR) stimulation, in vitro proliferation of CD4+ T cells was lower in Stat5b-CA TG versus WT mice, although because Treg-depleted CD4+ T-cell responders were not used, the influence of Stat5b-CA expression on Teffs could not be derived from these studies. Naive CD4+CD25−CD45RBhigh T cells from Stat5b-CA TG mice showed enhanced homeostatic proliferation when transferred into WT recipients10 and, in contrast to WT CD4+CD25−CD45RBhigh T cells, this proliferation was major histocompatibility complex (MHC) class II- and IL-15-independent, suggesting deregulation of homeostatic proliferation by Stat5b-CA in naive CD4+ T cells.10
Previous studies have not reported the in vivo function of Stat5b-CA TG Tregs. We show that Stat5b-CA TG Tregs suppressed graft-versus-host disease (GVHD) lethality more efficiently than WT Tregs. Surprisingly, Stat5b-CA TG CD4+CD25− Teffs also had a reduced GVHD lethality capacity compared with WT Teffs. Nonetheless, Stat5b-CA TG Teffs retained a graft-versus-leukemia (GVL) effect. These results indicate, for the first time, that Stat5 overexpression in CD4+ T cells plays a major role in Treg expansion and potency in vivo. Moreover, we provide the first evidence that Stat5 overexpression in CD4+ T cells regulates Th2 cytokine production in vivo that is associated with a reduction in GVHD. These studies suggest that Stat5b may be a target of pharmacologic up-regulation to prevent GVHD while retaining GVL for tumors not influenced by Stat5b signaling.
Methods
Mice
C57BL/6 (B6) Stat5b-CA TG mice were generated as described5 and offspring genotyped using polymerase chain reaction. FoxP3-GFP knock-in (FoxP3-GFP-KI) mice were provided by Dr Alexander Rudensky11 ; the mice were bred with Stat5b-CA TG mice and TG versus non-TG littermates were used for sorting experiments. B6-CD45.1 congenic, B10.BR, BALB/c, and B6(C)-H2-Ab1bm12/KhEgJ (bm12) mice were purchased from The Jackson Laboratory. B6 mice were purchased from the National Institutes of Health. Mice were housed in a specific pathogen–free facility in micro-isolator cages with University of Minnesota Institutional Animal Care and Use Committee approval.
Cell purification
CD4+ T cells were purified to more than 98% from lymph node (LN) cells incubated with phycoerythrin (PE)-conjugated antibodies against CD8, CD19, γδ-TCR, and NK1.1, then anti-PE magnetic beads, and the flow-through collected from magnetic depletion (LD) columns (Miltenyi Biotec). Tregs were isolated from CD4+ T cells by incubation with anti-CD25 PE monoclonal antibody, anti-PE beads, and repeated LS or MS column (Miltenyi Biotec) separation until CD25+ purity was more than 90%. Lamina propria lymphocyte isolation was as described.12,13 GFP−CD4+CD25− cells from FoxP3-GFP-KIxStat5b-CA or FoxP3-GFP-KI mice were flow-sorted.
In vitro assays
TG Teffs were activated with anti-CD3/28 beads (3 beads: 1 Teff) and IL-2 (1000 IU/mL), and CD25 expression analyzed after 5 or 18 hours. For suppression, Teffs (105 cells/well) were cultured with irradiated, T-cell-depleted bm12 stimulators at various Teff/Treg ratios in 96-well plates, tritiated thymidine added on day 6, and uptake was measured after 18 hours.
Flow cytometry
A total of 0.5 to 2 × 106 cells were incubated with antibodies for 20 to 30 minutes at 4°C. Cytokine staining was performed after incubation with αCD3ϵ antibody-coated plates (5 mg/mL) at 37°C and monensin (1:10 dilution, BD Biosciences) for 4 hours using BD Biosciences Cytofix/Cytoperm kit. Ki-67 and allophycocyanin-labeled annexin V kits (BD Biosciences) were used. PE-labeled counting beads were used for enumeration. Cells were acquired on a FACSCalibur. Analysis was done with FlowJo software Version 8.5.
GVHD and GVL models
Recipients were irradiated on day −1 (bm12: 800 cGy x-ray; B10.BR: 850 cGy x-ray or 1100 cGy 137cesium; BALB/c: 875cGy 137cesium). CD4+CD25− Teffs were mixed with 20 × 106 BM.12 Tregs were injected separately. For GVL studies, irradiated BALB/c recipients were given B6 BM plus or minus Stat5b-CA or WT CD4+CD25− Teffs (2 × 106); some cohorts were given BALB/c A20 lymphoma cells (3 × 105) coexpressing dsred2 and Renilla luciferase (termed A20luc).13 A total of 0.1 mL (60μM) EnduRen (Promega) substrate was injected intraperitoneally into mice and images collected on IVIS100.13 Recipient survival was monitored daily, and weights were recorded twice weekly.
CFSE labeling
CD4+CD25− or CD4+FoxP3− T cells (2.5 × 106 cells/mL) were incubated with 2.5μM carboxyfluorescein succinimidyl ester (CFSE)/mL for 2 to 3 minutes and 5 × 106 CFSE-labeled cells injected with 107 BM cells. Annexin V staining, responder frequency, and proliferation index were determined using FlowJo software Version 7.5.
Tissue analysis
Representative mice were killed on indicated days, and organs were embedded in OCT compound (Miles), snap frozen in liquid nitrogen, and stored at −80°C; 6-μm sections were cut, fixed in acetone, and hematoxylin and eosin–stained. Scoring was performed by A.P.-M. using a semiquantitative system of coded samples.14
Statistical analysis
The log-rank test was used for comparisons of survival. All other comparisons were done using a 2-sided unpaired t test. P values less than or equal to .05 were considered significant.
Results
Stat5b-CA TG CD4+ T cells have markedly reduced GVHD-inducing potential
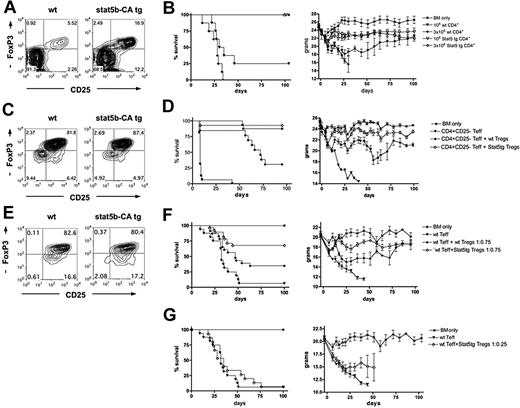
To investigate the role of Stat5 overexpression in CD4+ T-cell mediated GVHD mortality, 106 non–CD25-depleted CD4+ T cells were isolated from WT B6 or Stat5b-CA TG mice (Figure 1A). Stat5b-CA TG CD4+ T cells contained 16.9% CD25+FoxP3+ cells versus 5.5% in WT controls. B6 BM and CD4+ T cells were injected into MHC-mismatched, lethally irradiated B10.BR recipients. Mice receiving WT T cells showed clear clinical GVHD signs, and 75% died within 45 days. Mice receiving Stat5b-CA TG CD4+ T cells did not show GVHD signs, and all survived long-term (Figure 1B). When the CD4+ T-cell dose was increased to 3 × 106, WT CD4+ T-cell recipients died within 34 days, whereas Stat5b-CA TG recipients survived long-term (Figure 1B). Weight curves indicated that Stat5b-CA TG CD4+ T cells caused only a modest and transient GVHD-induced weight loss, in contrast to the striking reduction seen with WT CD4+ T cells (Figure 1B). These results indicate that Stat5b-CA TG CD4+ T cells have a reduced GVHD potential.
Effect of Stat5b overexpression on CD4 T-cell function in a GVHD model. (A) CD25 and FoxP3 expression of pooled CD4+ T cells purified from LNs of WT (left) and Stat5b-CA TG (right) mice, used for the experiment shown in panel B. (B) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1 × 106 (▼, ▽) or 3 × 106 (♦, ◇) CD4+ T cells isolated from WT (▼, ♦) or Stat5b-CA TG (▽, ◇) mice. P = .003, ▼ vs ▽. P < .001, ♦ vs ◇. (C) Values are for one experiment; n = 8 mice/group. CD25 and FoxP3 expression of CD4+CD25+ T cells purified from pooled LNs of WT (left) and Stat5b-CA TG (right) mice, used for the experiment shown in panel D. (D) Survival and weights of lethally irradiated bm12 recipient injected with 107 BM (*) and 2 × 106 B6 CD4+CD25− T cells alone (▼) or along with 2 × 106 CD4+CD25+ Tregs isolated from WT (♦) or Stat5b-CA TG (◇) mice. P = .001, ♦ vs ◇. P < .001, ▼ vs ♦ and ◇. Pooled data are from 2 experiments; n = 16 mice/group total. (E) CD25 and FoxP3 expression of purified CD4+CD25+ T cells isolated from pooled LNs of WT (left) and Stat5b-CA TG (right) mice, used for experiment shown in panel F. (F) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1.5 × 106 B6 CD4+CD25− T cells alone (▼) or along with 1.125 × 106 CD4+CD25+ Tregs isolated from WT (♦) or Stat5b-CA TG (◇) mice. P = .003, ♦ vs ◇. P = .02, ▼ vs ♦. P < .001, ▼ vs ◇. Pooled data are from 2 experiments; n = 16 mice/group total. (G) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1.5 × 106 B6 CD4+CD25− T cells alone (▼) or along with 0.375 × 106 CD4+CD25+ Tregs isolated from Stat5b-CA TG (◇) mice. P = not significant, ▼ vs ◇. Pooled data are from 2 experiments; n = 16 mice/group total.
Effect of Stat5b overexpression on CD4 T-cell function in a GVHD model. (A) CD25 and FoxP3 expression of pooled CD4+ T cells purified from LNs of WT (left) and Stat5b-CA TG (right) mice, used for the experiment shown in panel B. (B) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1 × 106 (▼, ▽) or 3 × 106 (♦, ◇) CD4+ T cells isolated from WT (▼, ♦) or Stat5b-CA TG (▽, ◇) mice. P = .003, ▼ vs ▽. P < .001, ♦ vs ◇. (C) Values are for one experiment; n = 8 mice/group. CD25 and FoxP3 expression of CD4+CD25+ T cells purified from pooled LNs of WT (left) and Stat5b-CA TG (right) mice, used for the experiment shown in panel D. (D) Survival and weights of lethally irradiated bm12 recipient injected with 107 BM (*) and 2 × 106 B6 CD4+CD25− T cells alone (▼) or along with 2 × 106 CD4+CD25+ Tregs isolated from WT (♦) or Stat5b-CA TG (◇) mice. P = .001, ♦ vs ◇. P < .001, ▼ vs ♦ and ◇. Pooled data are from 2 experiments; n = 16 mice/group total. (E) CD25 and FoxP3 expression of purified CD4+CD25+ T cells isolated from pooled LNs of WT (left) and Stat5b-CA TG (right) mice, used for experiment shown in panel F. (F) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1.5 × 106 B6 CD4+CD25− T cells alone (▼) or along with 1.125 × 106 CD4+CD25+ Tregs isolated from WT (♦) or Stat5b-CA TG (◇) mice. P = .003, ♦ vs ◇. P = .02, ▼ vs ♦. P < .001, ▼ vs ◇. Pooled data are from 2 experiments; n = 16 mice/group total. (G) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1.5 × 106 B6 CD4+CD25− T cells alone (▼) or along with 0.375 × 106 CD4+CD25+ Tregs isolated from Stat5b-CA TG (◇) mice. P = not significant, ▼ vs ◇. Pooled data are from 2 experiments; n = 16 mice/group total.
Stat5b-CA TG Tregs have a higher in vitro and in vivo suppressive potential than WT Tregs
Because the percentage CD4+ T cells considered to be Tregs is approximately 3-fold higher in Stat5b-CA TG than in WT mice, it is possible that the percentage Stat5b-CA TG Tregs was high enough to suppress GVHD induced by Teffs, although typically high Treg/Teff ratios are necessary for GVHD inhibition (Treg/Teff ratios of 1:1−3:1).15-17 Stat5b-CA TG Tregs may be more potent in suppressing alloresponses than WT Tregs. Alternatively, Stat5b-CA Teffs per se could be defective in inducing GVHD or be more sensitive to suppression by Tregs, resulting in less inflammation.
To determine whether Stat5b-CA TG versus WT Tregs were more suppressive on a per-cell basis, an in vitro suppression assay was performed using B6 WT Teffs and irradiated MHC class II-disparate (bm12) stimulators. TG Tregs decreased WT Teff proliferation less than 50% of control at ratios that were approximately 3-fold lower than WT Tregs (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Similarly, Stat5-CA TG Teffs were approximately 3-fold more readily suppressed by Stat5b-CA versus WT TG Teffs (supplemental Figure 1B). Thus, Stat5b-CA TG Tregs are more suppressive than WT Tregs on in vitro WT and TG Teff alloproliferation.
To assess Stat5b-CA TG Treg suppressor function in vivo, lethally irradiated recipients were injected with WT CD4+CD25− Teffs. Some cohorts also received freshly isolated WT or Stat5b-CA TG CD4+CD25+ Tregs (Figure 1C,E) at a Teff/Treg ratio, allowing for at least partial protection from GVHD by WT Tregs (1:1 for bm12 recipients; 1:0.75 for B10.BR recipients). WT and Stat5b-CA TG Treg purity was similar (81.8% WT vs 87.4% Stat5b-CA TG CD25+FoxP3+, Figure 1C; 82.8% WT vs 80.4% Stat5b-CA CD25+FoxP3+, Figure 1E). In bm12 recipients, WT Tregs rescued 31% of mice from lethal GVHD, significantly lower than the rescue of 94% of recipients of Stat5b-CA TG Tregs (Figure 1D). In B10.BR recipients, WT Teffs caused lethal GVHD in 94% of recipients (Figure 1F). The coinfusion of WT Tregs rescued 31% of recipients, significantly lower than the 69% of recipients of Stat5b-CA TG Tregs. Weight curves showed that Stat5b-CA Tregs were able to prevent the profound GVHD weight loss induced by WT Teffs, whereas the coinfusion of WT Tregs still resulted in a more than 20% weight loss in recipients of WT Teffs (Figure 1D,F). To determine whether a 3-fold lower ratio of Teff/Treg using Stat5-CA TG Tregs was sufficient to prevent GVHD lethality, cohorts of lethally irradiated B10.BR mice were given Stat5b-CA Tregs at Teff/Treg ratio of 1:0.25. At this ratio, Stat5b-CA TG Tregs failed to significantly suppress GVHD-induced lethality or weight loss by WT Teffs (Figure 1G).
The expression of relevant markers of Treg activation and function of freshly isolated CD4+FoxP3+ T cells were similar between WT and Stat5b-CA TG Tregs (Figure 2A). This indicates that differences in Treg potency between WT and Stat5b-CA TG Tregs were probably not the result of increased activation status of freshly isolated Stat5b-CA TG Tregs. Studies were performed to determine whether a higher percentage Stat5b-CA TG Tregs were present in the spleen, an important site of GVHD induction because the ratio of Teffs/Tregs probably contributes to the propensity for Tregs to suppress Teffs in situ. Lethally irradiated B10.BR mice were injected with congenic CD45.1+CD4+CD25− Teffs and WT or Stat5b-CA TG Tregs (both CD45.2). On day 10 after bone marrow transplantation (BMT), spleens were analyzed for CD45.2+CD4+ Tregs. No difference in the percentage Tregs was observed between groups (Figure 2B). The small intestine is an especially susceptible GVHD target organ in the B6 → B10.BR model,13 and our prior studies have indicated that Tregs present at that location are associated with decreased proinflammatory cytokine production at this site.13 Therefore, we determined the percentage Tregs and the cytokine profile of Teffs in the small intestine on day 10 after BMT. The percentage Tregs in recipients of Stat5b-CA TG Tregs was higher compared with WT Treg recipients (16.8% vs 11.8%; P < .002; Figure 2C), resulting in a significantly lower percentage Teffs in Stat5b-CA TG compared with WT Treg recipients (data not shown). The lower percentage Teffs in recipients of Stat5b-CA TG versus WT Tregs or no Tregs coincided with a significant reduction of the production of the GVHD effector cytokines interferon-γ (IFN-γ) and especially tumor necrosis factor-α (TNF-α; Figure 2D). These data suggest that a higher percentage Tregs and diminished proinflammatory cytokine production by Teffs infiltrating the small intestine contributed to the reduced GVHD injury and associated mortality in recipients of Stat5b-CA TG Tregs
Effect of Stat5b-CA TG expression on Treg phenotype homeostasis and suppressor function. (A) Flow cytometric analysis of fresh WT and Stat5b-CA TG LN CD4+FoxP3+ T cells incubated with monoclonal antibody specific for the indicated Ag; representative data from 1 of 2 mice per group are shown. The percentage donor CD4+CD45.2+ expressing T cells in the spleen (B) and small intestine (C) isolated from lethally irradiated B10.BR recipients injected with 107 BM and 106 WT CD45.1+CD4+CD25− T cells together with WT or Stat5b-CA TG CD45.2+CD4+CD25+ Tregs on day 10 after transplantation. (D) Expression of IFN-γ and TNF-α in CD45.1+CD4+ T cells isolated from the small intestine of lethally irradiated B10.BR recipients injected with 107 BM and 106 WT CD45.1+CD4+CD25− T cells with or without WT or Stat5b-CA TG CD45.2+CD4+CD25+ Tregs on day 10 after transplantation. (B-D) P values are as indicated. Data are from 1 experiment with 4 to 6 mice/group.
Effect of Stat5b-CA TG expression on Treg phenotype homeostasis and suppressor function. (A) Flow cytometric analysis of fresh WT and Stat5b-CA TG LN CD4+FoxP3+ T cells incubated with monoclonal antibody specific for the indicated Ag; representative data from 1 of 2 mice per group are shown. The percentage donor CD4+CD45.2+ expressing T cells in the spleen (B) and small intestine (C) isolated from lethally irradiated B10.BR recipients injected with 107 BM and 106 WT CD45.1+CD4+CD25− T cells together with WT or Stat5b-CA TG CD45.2+CD4+CD25+ Tregs on day 10 after transplantation. (D) Expression of IFN-γ and TNF-α in CD45.1+CD4+ T cells isolated from the small intestine of lethally irradiated B10.BR recipients injected with 107 BM and 106 WT CD45.1+CD4+CD25− T cells with or without WT or Stat5b-CA TG CD45.2+CD4+CD25+ Tregs on day 10 after transplantation. (B-D) P values are as indicated. Data are from 1 experiment with 4 to 6 mice/group.
Stat5b-CA TG versus WT CD4+CD25− Teffs have a higher responder frequency but undergo increased apoptosis resulting in reduced GVHD lethality
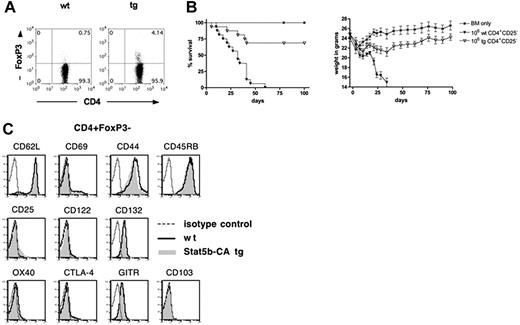
Although Stat5b-CA TG Tregs are more potent suppressors of GVHD than WT Tregs, the degree of GVHD inhibition in recipients of Stat5b-CA TG versus WT CD4+ T cells appeared out of proportion to the percentage Tregs in the donor graft. Moreover, there was an apparent discordance between the profound reduction in GVHD lethality potential seen using Stat5b-CA TG versus WT CD4+ T cells and both the partial GVHD lethality protection seen with a WT Teff/Stat5b-CA Treg ratio of 1:0.75 (Figure 1F) and lack of GVHD inhibition by a ratio of 1:0.25 (ie, 20% Stat5b-CA Tregs; Figure 1G), suggesting that Stat5b-CA TG expression had an influence on Teff function independent of Treg effects. To determine whether Stat5b-CA TG expression modifies Teff function, CD4+CD25− Teffs were isolated from WT or Stat5b-CA TG mice. The percentage CD4+CD25−FoxP3+ cells remaining after CD25 depletion was 0.8% versus 4.1%, respectively, consistent with the overall mean percentage FoxP3+ cells in CD4+CD25− Teffs purified using bead-based selection from Stat5b-CA versus WT donor was 3.3% versus 0.9%, respectively (n = 3 experiments; P = .01; Figure 3A; and data not shown). CD4+CD25− Teffs were injected into lethally irradiated B10.BR mice. Recipients of Stat5b-CA TG Teffs (106 cells) survived significantly longer than and had only a modest weight loss compared with those receiving WT Teffs (Figure 3B). The phenotype of naive Teffs from WT and Stat5b-CA TG mice was similar when analyzed for activation markers and cytokine receptors (Figure 3C). Thus, the profound GVHD survival results do not appear to be the result of differences in the activation status of infused Teffs from WT versus TG donors.
Effect of Stat5b-CA TG expression on Teff phenotype and effector function. (A) CD4 and FoxP3 expression of freshly purified and pooled CD4+CD25− T cells isolated from LNs of 10 WT (left) and 6 Stat5b-CA TG (right) mice. (B) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 2 × 106 CD4+CD25− T cells isolated from WT (▼) or Stat5b-CA TG mice (▽). P < .001, ▼ vs ▽. Pooled data are from 2 experiments; n = 16 mice/group total. (C) Flow cytometric analysis of freshly isolated WT and Stat5b-CA TG LN CD4+FoxP3− T cells incubated with monoclonal antibody specific for the indicated antigen; representative data from 1 of 2 mice per group are shown.
Effect of Stat5b-CA TG expression on Teff phenotype and effector function. (A) CD4 and FoxP3 expression of freshly purified and pooled CD4+CD25− T cells isolated from LNs of 10 WT (left) and 6 Stat5b-CA TG (right) mice. (B) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 2 × 106 CD4+CD25− T cells isolated from WT (▼) or Stat5b-CA TG mice (▽). P < .001, ▼ vs ▽. Pooled data are from 2 experiments; n = 16 mice/group total. (C) Flow cytometric analysis of freshly isolated WT and Stat5b-CA TG LN CD4+FoxP3− T cells incubated with monoclonal antibody specific for the indicated antigen; representative data from 1 of 2 mice per group are shown.
To explore the potential explanations for the decreased GVHD capacity of Stat5b-CA TG Teffs, Stat5b-CA TG CD4+CD25− Teffs, containing 4.4% CD4+25+, were activated with anti-CD3/28 monoclonal antibody-coated beads and IL-2. CD25 was reexpressed on 48% (5 hours) and 94% (18 hours) of Stat5b-CA TG CD4+CD25− T cells. These data indicated that CD4+CD25− TG Teffs were not defective in CD25 up-regulation and hence should be capable of responding to IL-2 signals in vivo (not shown). Nonactivated B6-CD45.1 Stat5b-CA TG CD4+CD25− Teffs were transferred into lethally irradiated B10.BR allogeneic recipients given B6 BM and splenocytes analyzed on day 13 after BMT. Thirty-seven percent of congeneic Teffs were CD4+CD25+; only 4.3% also expressed FoxP3 (not shown). These data indicated that CD25 expression is up-regulated in vivo; and on day 13 after BMT, the majority of the congenic splenic T cells were not Tregs.
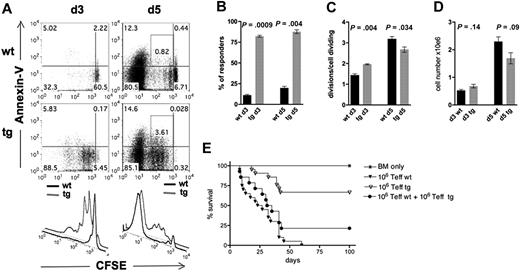
To directly determine whether Stat5b-CA TG Teffs were defective in their in vivo proliferation in response to host alloantigens, CFSE-labeled WT versus Stat5b-CA TG Teffs (5 × 106) were injected into lethally irradiated recipients. Splenocytes were isolated on days 3 and 5 after transplantation and analyzed for CFSE dilution and annexin V binding (Figure 4A). Whereas 82% of Teffs of Stat5b-CA TG donors in the spleen divided at least once after 3 days, significantly fewer WT Teffs (11%) had divided in the host at this time (Figure 4B). By day 5, the responder frequency remained significantly higher in TG versus WT Teffs (88% vs 20%, respectively; Figure 4B). The mean number of divisions of responders (proliferation index) of Stat5b-CA TG Teffs was significantly, albeit modestly, higher than of WT T cells on day 3 (2.0 ± 0.03 vs 1.4 ± 0.07) and significantly, but modestly lower (2.7 ± 0.13 vs 3.2 ± 0.12), on day 5 after BMT (Figure 4C). A higher frequency of donor CD4+ T cells from Stat 5-CA TG versus WT recipients underwent less than or equal to four cell divisions and a higher fraction of cells undergoing one to four divisions were annexin V positive (0.8% ± 0.03% vs 3.0% ± 0.39%; P = .006, Figure 4A; and data not shown). The net result was a comparable splenocyte number in recipients of Stat5b-CA TG Teffs on day 3 after BMT and a trend (P = .09) toward a lower Teff number on day 5 after BMT (Figure 4D).
CFSE dilution and annexin V binding of CD4+CD25− T cells isolated from spleens of lethally irradiated B10.BR recipients injected with 107 BM and 5 × 106 CFSE-labeled CD4+CD25+ T cells from WT or Stat5b-CA TG mice. (A) Splenocytes were isolated on day 3 (left) and day 5 (right) after transplantation, and donor H-2Kb+CD4+ T cells were analyzed for CFSE dilution (illustrated in top and bottom panels) and annexin V (illustrated in top panels) expression. Representative flow cytometry data from one of 6 mice/group from 2 separate experiments are shown. Responder frequency (B) and proliferation index (C) were calculated. (D) Total cell numbers harvested from spleen on day 3 and day 5 from recipients described in panel A. (E) Survival of lethally irradiated B10.BR recipients of 107 BM (*) and 1 × 106 B6 CD4+CD25− T cells isolated from WT (▼) or Stat5b-CA TG (▽) mice or a mixture of both (●). P = not significant for ▼ vs ●. P < .01, ▼ and ● vs ▽. Pooled data are from 2 experiments; n = 16 mice/group total.
CFSE dilution and annexin V binding of CD4+CD25− T cells isolated from spleens of lethally irradiated B10.BR recipients injected with 107 BM and 5 × 106 CFSE-labeled CD4+CD25+ T cells from WT or Stat5b-CA TG mice. (A) Splenocytes were isolated on day 3 (left) and day 5 (right) after transplantation, and donor H-2Kb+CD4+ T cells were analyzed for CFSE dilution (illustrated in top and bottom panels) and annexin V (illustrated in top panels) expression. Representative flow cytometry data from one of 6 mice/group from 2 separate experiments are shown. Responder frequency (B) and proliferation index (C) were calculated. (D) Total cell numbers harvested from spleen on day 3 and day 5 from recipients described in panel A. (E) Survival of lethally irradiated B10.BR recipients of 107 BM (*) and 1 × 106 B6 CD4+CD25− T cells isolated from WT (▼) or Stat5b-CA TG (▽) mice or a mixture of both (●). P = not significant for ▼ vs ●. P < .01, ▼ and ● vs ▽. Pooled data are from 2 experiments; n = 16 mice/group total.
Decreased GVHD lethality of Stat5b-CA TG CD4+CD25− Teffs is the result of both residual Tregs that have increased suppressor potency and an intrinsic defect in Stat5b-CA TG Teff function
Given the reduced lethality seen with Stat5b-CA Teffs, we next focused on the possible suppressor cell function of Stat5b-CA CD4+CD25− Teffs. Lethally irradiated recipients were injected with 106 CD4+CD25− Teffs from WT or Stat5b-CA TG mice or 106 each from both WT and Stat5b-CA TG Teffs. In concordance with earlier results, recipients of WT T cells died within 60 days, whereas most recipients of Stat5b-CA TG Teff cells survived long-term (Figure 4E). Recipients of WT and Stat5b-CA TG Teffs showed a survival similar to recipients of WT T cells alone, indicating that Stat5b-CA TG Teffs do not suppress WT Teff function (Figure 4E).
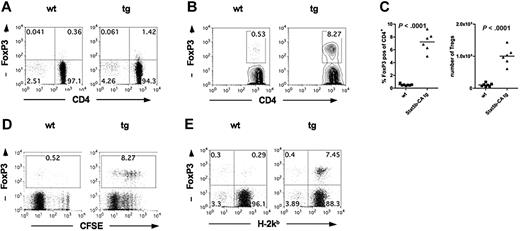
One explanation for reduced GVHD seen after Stat5b-CA TG Teffs is the presence of higher residual CD4+FoxP3+ cells in the Stat5b-CA TG versus WT Teff fraction (4.1% vs 0.8%; Figure 3A), although the FoxP3 mean fluorescent intensity (MFI) was less than 100 in the residual Tregs from both the WT and TG donors, at least 2-fold lower than their corresponding non–CD25-depleted CD4+ T-cell fractions (eg, Figure 3A vs Figure 1A). Because FoxP3 MFI correlates with suppressor potency,18 these data suggested that the remaining Tregs had reduced suppressor cell potency on a per-cell basis compared with Tregs in the non–CD25-depleted CD4+ T-cell fraction. Nonetheless, we could not exclude the possibility that contaminating Tregs in Stat5b-CA TG versus WT CD4+CD25− Teffs had an in vivo proliferative advantage, leading to a physiologically relevant change in Teff/Treg ratios in vivo. Therefore, studies were performed to determine the percentage and absolute Treg numbers in the spleen after BMT in recipients of CD4+CD25− Teffs from Stat5b-CA TG versus WT donors. CFSE-labeled CD4+CD25− Teffs from WT or Stat5b-CA TG donors were injected into lethally irradiated B10.BR recipients. The baseline percentage FoxP3+ cells in donor CD4+CD25− Teffs was 0.4% in the WT (Figure 5A); and after 5 days, the percentage donor FoxP3+ Tregs in the spleen was 0.50% plus or minus 0.02% (Figure 5B-C). Whereas the baseline percentage FoxP3+ cells was 1.4% in the Stat5b-CA TG CD4+CD25− Teff inoculum (Figure 5A), the percentage donor FoxP3+ Tregs in the spleen on day 5 was 6.6% plus or minus 0.65% (Figure 5B-C), proportionately higher than the WT Tregs even after correcting for differences in the percentage FoxP3+ input cells. Thus, although there was approximately 3.5 more CD4+FoxP3+ cells in Stat5b-CA TG versus WT donor inoculum, by day 5 after BMT, approximately 10-fold more CD4+FoxP3+ cells were seen in the spleen of recipients of TG versus WT Tregs, representing approximately 3-fold greater in vivo expansion of TG versus WT Tregs. Moreover, in contrast to FoxP3 MFI in the donor inoculum of CD4+CD25− Teffs, the FoxP3 MFI of Tregs in the spleen of recipients of WT and Stat5b-CA TG Teffs was at least 2-fold higher.
Stat5b-CA TG expression supports Treg proliferation after transplantation into allogeneic hosts. (A) CD4 and FoxP3 expression of purified CD4+CD25− T cells isolated from lymph nodes of WT (left) and Stat5b-CA TG (right) mice. Pooled data are from the experiment shown in panel B; n = 10/group. (B) Lethally irradiated B10.BR recipients were injected with 107 BM cells and 5 × 106 CFSE-labeled CD4+CD25− T cells from WT or Stat5b-CA TG mice. (B-C) Splenocytes were isolated on day 5 after transplantation, and the percentage and absolute number of CD4+H-2Kb+FoxP3+ WT and Stat5b-CA TG T cells were determined. P values are as indicated. (D) The CFSE dilution profile was determined. (E) The percentage host (H-2Kb−CD4+FoxP3+) and percentage donor (H-2Kb+CD4+FoxP3+) T cells were analyzed. (B-E) Representative data from one mouse or pooled data from 2 independent experiments of 6 or 7 mice/group total are shown.
Stat5b-CA TG expression supports Treg proliferation after transplantation into allogeneic hosts. (A) CD4 and FoxP3 expression of purified CD4+CD25− T cells isolated from lymph nodes of WT (left) and Stat5b-CA TG (right) mice. Pooled data are from the experiment shown in panel B; n = 10/group. (B) Lethally irradiated B10.BR recipients were injected with 107 BM cells and 5 × 106 CFSE-labeled CD4+CD25− T cells from WT or Stat5b-CA TG mice. (B-C) Splenocytes were isolated on day 5 after transplantation, and the percentage and absolute number of CD4+H-2Kb+FoxP3+ WT and Stat5b-CA TG T cells were determined. P values are as indicated. (D) The CFSE dilution profile was determined. (E) The percentage host (H-2Kb−CD4+FoxP3+) and percentage donor (H-2Kb+CD4+FoxP3+) T cells were analyzed. (B-E) Representative data from one mouse or pooled data from 2 independent experiments of 6 or 7 mice/group total are shown.
Proliferation analysis using CFSE dilution showed that most Stat5b-CA TG and WT Tregs had divided by day 5 (Figure 5D). Although a higher frequency of TG versus WT Teffs (FoxP3− cells) underwent multiple divisions, the proportion of donor cells expressing FoxP3 was higher in TG than WT Teffs, resulting in a higher Treg/Teff ratio. On day 5 after BMT, splenic CD4+ cells were 96% donor in origin in both groups. However, WT FoxP3+ T cells were approximately 50% donor and 50% host origin and totaled only 0.6% of the spleen (Figure 5E). In contrast, Stat5b-CA TG FoxP3+ T cells were 95% donor origin and totaled 7.9% of the spleen, indicating that Stat5b overexpression in donor CD4+ T cells was responsible for the increased percentage Tregs observed in vivo. Together, these data suggested that donor Stat5b-CA TG Tregs have a proliferative advantage over WT Tregs in relation to Teffs under the prevailing GVHD conditions that result in a higher Treg/Teff ratio compared with WT recipients.
Although the data shown in Figure 5 provided significant insight as to the in vivo proliferation and persistence of small numbers of Tregs in Stat5b-CA TG CD4+CD25− Teffs, the GVHD potential of TG Teffs alone was difficult to derive from these studies. Therefore, experiments were performed to rigorously deplete Tregs from the CD4+CD25− Teff inoculum. CD4+CD25− T cells from B6 FoxP3-GFP-KI mice or FoxP3-GFP-KI × Stat5b-CA TG mice, which have Tregs marked by GFP expression, were sorted to a purity of 99.8% GFP− cells (data not shown), virtually eliminating all Tregs without adversely affecting their function as would be the case if FoxP3 staining was used for sorting. Recipients of FoxP3-GFP-KI × Stat5b-CA TG GFP−CD4+FoxP3− Teffs survived significantly longer than recipients of FoxP3-GFP-KI Teffs, further substantiating a Teff intrinsic defect in Stat5b-CA TG cells (Figure 6A). A significant decrease of GVHD was noted in the small intestine (mean scores of 4.0-2.6), albeit with increased yet still low-grade tissue scores (mean scores of < 1.5) in the liver, lung, and pancreas (Figure 6B).
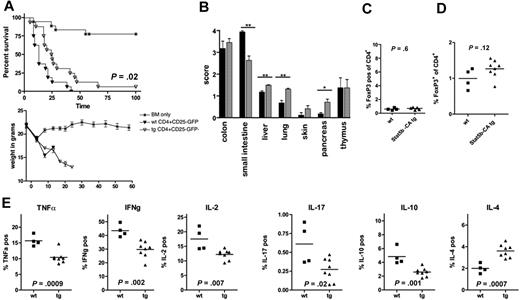
Stat5b-CA TG expression impairs intrinsic Teff function but does not induce a Treg phenotype. (A) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1 × 106 sorted CD4+CD25−GFP− T cells from FoxP3-GFP-KI (WT; ▼) or FoxP3-GFP-KI × Stat5b-CA TG mice (TG; ▽) mice. P = .02, ▼ vs ▽. Pooled data are from 2 experiments; n = 16 mice/group total. (B) Histologic grade of GVHD in organs indicated on day 10 after transplantation in FoxP3-GFP-KI (WT; black) and FoxP3-GFP-KI × Stat5b-CA TG (Stat5b-CA TG; gray) recipients. **P < .01. *P < .05. Data are from 1 experiment with 4 and 7 or 8 mice/group. Splenocytes (C) and lamina propria cells (D) of recipients described in panel A were isolated on day 10, and the percentage FoxP3+(GFP+) cells was determined (panel C, 1 experiment with 4 and 5; and panel D, 4 and 8 mice per group, respectively). P values are as indicated. (E) The percentage TNF-α, IL-2, IFN-γ, IL-10, IL-17, or IL-4 expressing lamina propria CD4+ T cells isolated from the small intestine on day 10 after transplantation. P values are as indicated. Data are from 1 experiment with 4 and 7 or 8 mice/group.
Stat5b-CA TG expression impairs intrinsic Teff function but does not induce a Treg phenotype. (A) Survival and weights of lethally irradiated B10.BR recipients injected with 107 BM (*) and 1 × 106 sorted CD4+CD25−GFP− T cells from FoxP3-GFP-KI (WT; ▼) or FoxP3-GFP-KI × Stat5b-CA TG mice (TG; ▽) mice. P = .02, ▼ vs ▽. Pooled data are from 2 experiments; n = 16 mice/group total. (B) Histologic grade of GVHD in organs indicated on day 10 after transplantation in FoxP3-GFP-KI (WT; black) and FoxP3-GFP-KI × Stat5b-CA TG (Stat5b-CA TG; gray) recipients. **P < .01. *P < .05. Data are from 1 experiment with 4 and 7 or 8 mice/group. Splenocytes (C) and lamina propria cells (D) of recipients described in panel A were isolated on day 10, and the percentage FoxP3+(GFP+) cells was determined (panel C, 1 experiment with 4 and 5; and panel D, 4 and 8 mice per group, respectively). P values are as indicated. (E) The percentage TNF-α, IL-2, IFN-γ, IL-10, IL-17, or IL-4 expressing lamina propria CD4+ T cells isolated from the small intestine on day 10 after transplantation. P values are as indicated. Data are from 1 experiment with 4 and 7 or 8 mice/group.
The combined data (Figures 2B-D, 3, and 5) indicate that both a diminished GVHD potential of Stat5b-CA Teffs and the presence of small numbers of highly proliferative CD4+CD25+FoxP3+ cells in the infused donor Stat5b-CA TG CD4+CD25− Teffs inoculum accounted for the reduced GVHD lethality seen using Stat5b-CA TG CD4+CD25− Teffs in earlier experiments (Figure 5A vs Figure 3B). Consistent with a contribution of residual Tregs to reduced GVHD potential seen in recipients of TG versus WT Teffs, the percentage Tregs in the spleen of day 10 after BMT recipients of FoxP3-GFP-KI and FoxP3-GFP-KI × Stat5b-CA TG GFP−CD4+CD25− Teffs was low (≤ 1%) in both groups (Figure 6C), in contrast to the higher frequency seen using non–sort-purified CD4+CD25− Teffs (Figure 5C). Importantly, these data also suggest that Stat5b-CA Teffs are not converted into Tregs from FoxP3− precursors at least as measured in the spleen on day 10 after BMT. Moreover, there was no difference of the percentage Tregs in the small intestine when analyzed on day 10 after BMT in mice receiving FoxP3-GFP-KI versus FoxP3-GFP-KI × Stat5b-CA TG GFP−CD4+CD25− Teffs (Figure 6D). Together, these data indicate that after BMT Tregs were derived from infused classic Tregs and not converted Teffs.
To characterize the Teff phenotype in these recipients, lamina propria infiltrating cells from recipients of FoxP3-GFP-KI × Stat5b-CA TG versus FoxP3-GFP-KI, CD4+CD25−GFP− Teffs were restimulated in vitro for 4 hours with anti-CD3e antibody and then analyzed by intracellular cytokine staining. Lamina propria cells from recipients of FoxP3-GFP-KI × Stat5b-CA TG had a lower percentage TNF-α, IL-2, IFN-γ, IL-10, and IL-17 expressing cells and a higher frequency of IL-4 expressing cells than seen in recipients of FoxP3-GFP-KI (Figure 6E). The mean donor CD4+ T-cell chimerism level was 99% in both groups (data not shown). These results show that overexpression of Stat5b leads to impaired generation of important regulators of GVHD lethality and demonstrate, for the first time, in vivo skewing of the immune response toward a Th2 (IL-4) phenotype that has been associated with improved survival in this model of GVHD.13
Stat5b-CA TG Teffs have a reduced GVHD capacity with retention of a GVL effect
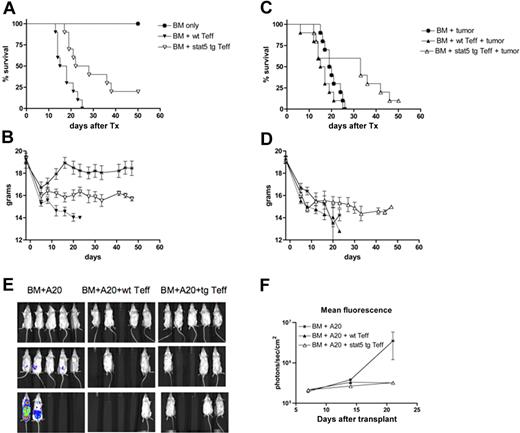
To determine whether the reduction in GVHD lethality by Stat5b-CA TG Teffs was of a sufficient magnitude or involved a critical T-cell subset essential for conferring a GVL effect, we used an A20 lymphoma model known to be sensitive to CD4+ Teff-mediated elimination. Irradiated BALB/c recipients were given BM with or without Stat5b-CA TG versus WT CD4+CD25− Teffs (2 × 106). Other cohorts received A20luc cells (3 × 105) on day 0. Stat5b-CA TG Teffs resulted in a significant reduction of GVHD induced mortality and weight loss compared with those receiving WT Teffs (Figure 7A-B). Stat5b-CA TG Teffs were capable of eliminating A20luc cells to the same extent as WT Teffs (Figure 7C-F). These data are in marked contrast to the BM only group, which succumbed to A20 lymphoma and the WT Teff group, which succumbed to GVHD lethality. Thus, the net result of infusing Stat5b-CA TG Teffs was a significant prolongation in survival because of reduced GVHD lethality and a retained GVL effect, probably reflecting the antileukemia effects of alloreactive Stat5b-CA TG CD4+CD25− Teffs.
Lethally irradiated B10.BR recipients were injected with 107 BM (*) and 1 × 106 CD4+CD25− T cells from WT (▼) or Stat5b-CA TG (●) mice. Mice were not challenged with A20 lymphoma cells and were monitored exclusively for GVHD lethality (A) and weights (B). Other cohorts of mice treated as in panel A also were given 105 A20 lymphoma cells on day 0 of BMT and were then monitored for survival (C) and weights (D). P values are as indicated. Data are from 1 experiment with 10 mice per group. Separate cohorts of mice were analyzed for bioluminescent imaging on days 7, 14, and 21. Data shown are the bioluminescence emissions (E) and the quantification of bioluminescence based on photons/sec/cm2 emission (y-axis) and days after BMT (x-axis; F). The SD of the mean is also shown.
Lethally irradiated B10.BR recipients were injected with 107 BM (*) and 1 × 106 CD4+CD25− T cells from WT (▼) or Stat5b-CA TG (●) mice. Mice were not challenged with A20 lymphoma cells and were monitored exclusively for GVHD lethality (A) and weights (B). Other cohorts of mice treated as in panel A also were given 105 A20 lymphoma cells on day 0 of BMT and were then monitored for survival (C) and weights (D). P values are as indicated. Data are from 1 experiment with 10 mice per group. Separate cohorts of mice were analyzed for bioluminescent imaging on days 7, 14, and 21. Data shown are the bioluminescence emissions (E) and the quantification of bioluminescence based on photons/sec/cm2 emission (y-axis) and days after BMT (x-axis; F). The SD of the mean is also shown.
Discussion
In the present study, we show that Tregs in Stat5b-CA TG mice have a superior suppressor function on a per-cell basis compared with WT Tregs as assessed in an in vivo GVHD model. No obvious differences in Treg activation status at baseline were seen between Stat5b-CA TG and WT Tregs. A small population of Stat5b-CA TG versus WT Tregs proliferated more extensively in vivo, resulting in a higher Treg/Teff cell ratio in situ. Stat5b-CA TG Tregs may have an advantage in trafficking to lymphoid and target organs of GVHD, as suggested by the differential accumulation of Tregs in the small intestine but not the spleen (Figure 2B-C). Despite augmented in vivo proliferation early after BMT, Stat5b-CA Teffs had a reduced GVHD lethality capacity associated with increased apoptosis, decreased Teff proinflammatory cytokines, and increased IL-4 production.
Several potential explanations exist that may account for the superior suppressor cell function of Stat5b-CA TG versus WT Tregs. Although FoxP3 mRNA is higher in Stat5b-CA TG versus WT Tregs,5 our flow cytometric studies did not reveal FoxP3 protein levels to be elevated; therefore, higher FoxP3 protein levels do not appear to provide an explanation for the augmented potency of TG versus WT Tregs. Superior Stat5b-CA Treg function also could be based on a different TCR repertoire with more alloantigen-specific Tregs that become activated and suppress a wider range of Teffs. Indeed, Stat5b-CA TG Tregs have a broader range of antigen specificity than WT Tregs.19 One study showed that transforming growth factor-β specifically interfered with Stat5-induced expression of genes involved in proliferation while preserving expression of antiapoptotic genes in CD8+ T cells.20 One could envision a similar regulation in Tregs to maintain an anergic phenotype and increased survival, despite activated Stat5. In the context of an infection challenge, activated T cells up-regulate CD25 and become more sensitive to cell death on TCR religation.21 Clearly, Tregs somehow escape this mechanism. CTLA-4, another molecule constitutively expressed in Tregs, was found to be able to interact with Stat5 and down-regulated Stat5 function in a T-cell line in vitro.22 Together, these studies provide some clues how Tregs can maintain their phenotype and survive long-term, despite their requirement for activated Stat5.
Surprisingly, Stat5b-CA TG Teffs proved to be less effective in inducing GVHD lethality than WT Teffs. We had expected increased lethality as a result of the anticipated higher proliferative potential and better survival of CD4+CD25− Teff cells because of the increased expression of antiapoptotic molecules.5 A previous study showed decreased rejection by Stat5αβ−/− T cells in a heart allograft model, which would suggest a state of increased rejection when Stat5 activation is enhanced.10 Because of the memory phenotype of CD8+ T cells in Stat5b-CA TG mice, we could not adequately evaluate their function in GVHD. Although a difference in TCR repertoire could be an explanation for the GVHD results seen using CD4+CD25− Teffs, this seems unlikely because Stat5b-CA TG Teffs had a repertoire similar to WT Teff cells.19 Our CFSE data show that the majority of Stat5b-CA Teffs undergo proliferation early after infusion, suggesting that not all proliferation was antigen-driven. As shown previously, Stat5b-CA Teff cells undergo extensive lymphopenia-induced and homeostatic proliferation, which is antigen-independent.10 Such bystander proliferation could result in competition of resources (eg, prosurvival cytokines or antigen-presenting cells) with alloantigen-specific Teffs and hence an overall decreased cytokine production by Stat5b-CA TG Teffs, although this explanation is not supported by the observation that Stat5b-CA added to WT Teffs had no difference in GVHD lethality compared with either population alone.
Rather, Stat5b-CA TG Teffs that were undergoing rapid division had higher annexin V staining than WT Teffs on day 5 after BMT, resulting in a modestly lower Teff number, consistent with increased sensitivity to cell death on TCR ligation.21 IL-2R signaling can actively down-regulate IL-2 cytokine production in a Stat5-dependent manner, and constitutive activation of Stat5b could induce a similar negative feedback signal in our system.23 Stat5 has been shown to be important for in vitro Th2 differentiation.24 Our studies prove, for the first time, that Stat5b overexpression increases the frequency of Th2 cytokine-producing cells in vivo, potentially contributing to reduced GVHD lethality by TG versus WT Teffs. In studies using Stat5-deficient mice, Stat5 has been implicated in inhibiting Th17 T-cell development in vitro and conversely supporting Treg generation in the presence of transforming growth factor-β.7 Previous studies have not shown that the converse is true (ie, that Stat5b overexpression can down-regulate Th17 cytokine production in vivo). Our present studies prove that Stat5b overexpression inhibits Th17 cytokine production in vivo during GVHD. The severity of CD4+ T-cell mediated GVHD injury has been linked to the frequency of IFN-γ, IL-17, and inversely to the frequency of IL-4-producing cells.12,13,25-28 However, other studies using cytokine knockout donors have demonstrated contrasting results, perhaps because of the pronounced skewing of cytokine-producing donor T cells and pattern of GVHD parenchymal organ injury.29-33 Although we could not find evidence for the conversion of Stat5b-CA TG Teffs into Tregs in the spleen or the small intestine on day 10 after BMT, we cannot exclude the possibility that conversion is favored in Stat5b-CA TG Teffs in other sites or at later time points after BMT. Increased peripheral Treg conversion from Stat5b-CA TG versus WT Teffs might have been predicted by the fact that thymic Treg progenitors from STAT5b-CA mice convert into mature Tregs much better than WT Treg progenitors.19
A GVL effect was observed despite decreased GVHD lethality in recipients of Stat5b-CA TG versus WT CD4+CD25− Teffs. We speculate that the residual alloresponse seen when Stat5b-CA CD4+CD25− Teffs are given is sufficient for reducing, but not eliminating, GVHD lethality and controlling of host-type A20 lymphoma cells. Host alloantigen recognition probably accounted for the elimination of A20luc cells by Stat5b-CA TG Teffs to a similar extent as WT Teffs. Future studies will need to be performed to determine the mechanisms responsible for the GVL effect and the level of donor Tregs sufficient to reduce GVHD and preserve GVL. This fine balance may be possible because the threshold for Teffs to clear lymphoma may be less than needed for GVHD, as supported by our prior studies targeting the IL-21 pathway.13
In conclusion, Stat5b activation can be enhanced to improve Treg suppressor function and simultaneously inhibit CD4+CD25− Teffs. Our studies suggest that pharmacologic up-regulation of Stat5b in Tregs or Teffs may be useful for GVHD prevention or therapy. Such an approach could be accomplished by ex vivo genetic modification of donor CD4+ Teffs or Tregs, permitting Stat5b induction in vivo when and for the duration needed, avoiding the potential tumorigenicity of constitutive Stat5b overexpression. Although less than 2% of Stat5b-CA TG mice developed B-cell lymphomas,5 some types of leukemia cells (eg, human acute myeloid leukemia) depend on the Stat5 pathway for survival signals.34,35 Nonetheless, even transient pharmacologic up-regulation of Stat5b expression in donor Teffs, as could be accomplished ex vivo before infusion along with the hematopoietic cell graft or as delayed lymphocyte infusions at the time of tumor recurrence may reduce GVHD lethality and retain a GVL effect. In addition, inducible, transient Stat5b up-regulation in Tregs could prove useful in preventing or controlling GVHD or autoimmunity. Preclinical studies in these areas will need to await the availability of small molecule or pharmacologic agents that specifically up-regulate Stat5b.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Children's Cancer Research Fund and the National Institutes of Health (R01 AI34495, HL56067, and CA72669). C.B. was supported in part by the Swiss National Science Foundation (PBBSB-108600) and Oncosuisse (BIL KLS 01617-12-2004). M.A.F. is a Leukemia & Lymphoma Society Investigator.
National Institutes of Health
Authorship
Contribution: C.V. and C.B. designed, conducted, and analyzed research and wrote the paper; E.G., S.L.H., L.K.K., and P.A.T. conducted research; M.A.F provided unique reagents and insights; A.P.-M. provided data; and B.R.B. designed research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
Author notes
C.V. and C.B. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal