To the editor:
A recent article by Hazan-Halevy et al assessed down-regulation of STAT3 in B-CLL cells (B-CLLs) using VSV-G pseudotyped lentiviral vectors (VSV-G-LVs) encoding for STAT3–small hairpin RNA (shRNA).1 They reported a transduction of 40% to 70% with VSV-G-LVs in nonstimulated B-CLLs. This is in sharp contrast with our recently published data demonstrating that VSV-G-LVs are not suited for efficient transduction of malignant B cells. Indeed, 4 independent laboratories reported that human B cells, even upon a proliferative stimulus, are highly refractory to VSV-G-LV–mediated gene transfer.2-5 We confirmed that even upon BCR stimulation, B-CLLs were not efficiently transduced by VSV-G-LVs as reported earlier by Bovia et al.2 Of high importance, we engineered a new generation of LVs carrying at their surface glycoproteins of the measles virus, H and F (H/F-LVs). These new LV-pseudotypes allowed very efficient gene transfer in resting T and B cells as well as in B-CLLs, whereas VSV-G-LVs failed, even at high vector doses.3,6
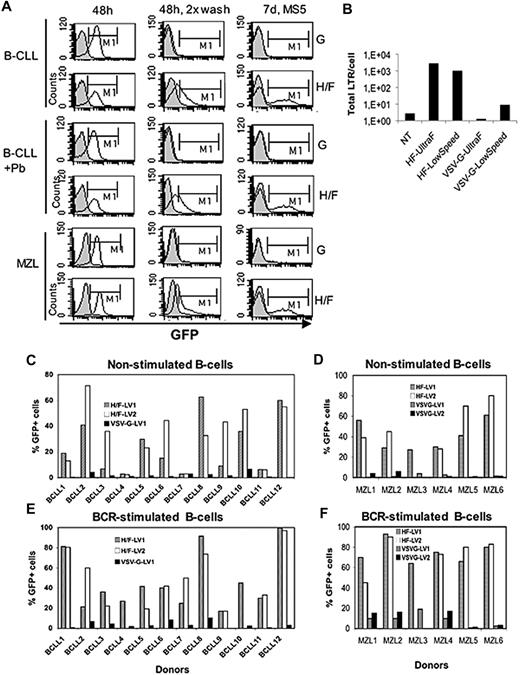
To understand the discrepancy between our results and those of Hazan-Halevy and colleagues,1 we produced green fluorescence protein (GFP)–encoding VSV-G- and H/F-LVs by ultrafiltration as reported by these authors. We transduced primary B-CLLs7 and another B-cell malignancy, marginal zone lymphoma cells (MZLs)8 with volumes of vectors concentrated by ultrafiltration and cell densities equivalent to those of Hazan-Halevy et al.1 Upon 48 hours' transduction, we evaluated the green fluorescence of the cells by FACS analysis (Figure 1A). We clearly detected a shift of transduced cells (H/F or VSV-G-LVs), to a higher mean fluorescence intensity in GFP channel similar to the shift observed by Hazan-Halevy et al. Surprisingly though, 2 consecutive washes of the cells after transduction reduced this GFP+ signal almost completely to background levels for VSV-G-LV transduction, whereas a residual GFP+ population remained visible upon H/F-LV transduction (Figure 1A). The same picture was found when polybrene was added during transduction. Moreover, when continuing the B-CLL and MZL cultures for 7 days on MS5 cells in the presence of cytokines, only HF-LV transduction resulted in stable GFP expression, whereas no GFP was detected for VSV-G-LV transduction (Figure 1A). Moreover, we confirmed a very low level, close to background, of reverse transcribed (RT) proviral DNA in VSV-G-LV–transduced cells, whereas a 100- to 1000-fold higher RT-DNA level was detected in H/F-LV–transduced cells (Figure 1B), again confirming unproductive VSV-G-LV transduction.
HF-LVs are highly superior for transduction of cancer B cells as compared with VSV-G-LVs. (A) Isolated chronic lymphocytic leukemia B cells (B-CLL) or peripheral marginal zone lymphoma B cells (MZL) were transduced with 100 μL of concentrated VSV-G-LV (MOI 300) or H/F-LV (MOI 3) viral supernatant. Both vectors were titrated on 293T cells. Vectors were concentrated by ultrafiltration-centrifugation as described in Hazan-Halevy et al.1 For the B-CLL donor cells, transductions were performed in parallel in the presence of polybrene (Pb; 10 ng/mL). At 48 hours upon transduction, cells were evaluated for green fluorescence by fluorescence-activated cell sorting (FACS) analysis without washing (48 hours) or followed by 2 subsequent washes with phosphate-buffered saline before FACS analysis (48 hours, 2× wash). Additionally, at 48 hours of transduction, part of the B cells were washed and were maintained on MS5 stromal cells in RPMI medium supplemented with 10% fetal calf serum, IL-2, and IL-15 to confirm stable transduction. At 7 days upon culture, the cells were harvested, passed through a mesh, and stained with anti-CD19APC. Cells were then evaluated for GFP expression by FACS analysis (MS5, 7 days). Data are representative of 3 different donors. (B) Transduction of MZL cells was performed with concentrated H/F- or VSV-G-LVs as mentioned. Two different concentration methods were used: (1) low-speed concentration (3000g, 4°C overnight, 100× concentration) or (2) ultrafiltration-centrifugation (UltraF) as performed by Hazan-Halevy et al.1 For VSV-G- and HF-LV transductions, cells were harvested at 48 hours and total levels of reverse transcribed proviral DNA (total LTR/cell) were determined by quantitative polymerase chain reaction (see “Methods” in Frecha et al3 ). Freshly isolated, nonstimulated B cells of different B-CLL (C) and MZL (D) donors were transduced with 2 different preparations of H/F-LVs (MOI 5, HF-LV1 and HF-LV2, low-speed concentration) or with VSV-G-LVs (MOI 50, VSV-G-LV1, VSV-G-LV2, low-speed concentration) for 48 hours. Cells were washed subsequently and maintained in culture on MS5 stromal cells in RPMI supplemented with 10% fetal calf serum, IL-2, and IL-15 for 5 more days before FACS analysis was performed as in panel A. (E-F) The same transduction protocols as described for panels C and D were applied on B-CLL and MZL cells prestimulated for 48 hours with Staphylococcus aureus Cowen and IL-2 (10 ng/mL). Note that for non- and BCR-stimulated MZL-3 cells, transduction was performed with only the H/F-LV1 vector preparation. Similarly, prestimulated B-CLL-4 and B-CLL-10 cells were transduced with only H/F-LV1. FACS analysis was performed 2 days after transduction followed by 5 days of culture on MS5 cells to allow evaluation of stable transduction.
HF-LVs are highly superior for transduction of cancer B cells as compared with VSV-G-LVs. (A) Isolated chronic lymphocytic leukemia B cells (B-CLL) or peripheral marginal zone lymphoma B cells (MZL) were transduced with 100 μL of concentrated VSV-G-LV (MOI 300) or H/F-LV (MOI 3) viral supernatant. Both vectors were titrated on 293T cells. Vectors were concentrated by ultrafiltration-centrifugation as described in Hazan-Halevy et al.1 For the B-CLL donor cells, transductions were performed in parallel in the presence of polybrene (Pb; 10 ng/mL). At 48 hours upon transduction, cells were evaluated for green fluorescence by fluorescence-activated cell sorting (FACS) analysis without washing (48 hours) or followed by 2 subsequent washes with phosphate-buffered saline before FACS analysis (48 hours, 2× wash). Additionally, at 48 hours of transduction, part of the B cells were washed and were maintained on MS5 stromal cells in RPMI medium supplemented with 10% fetal calf serum, IL-2, and IL-15 to confirm stable transduction. At 7 days upon culture, the cells were harvested, passed through a mesh, and stained with anti-CD19APC. Cells were then evaluated for GFP expression by FACS analysis (MS5, 7 days). Data are representative of 3 different donors. (B) Transduction of MZL cells was performed with concentrated H/F- or VSV-G-LVs as mentioned. Two different concentration methods were used: (1) low-speed concentration (3000g, 4°C overnight, 100× concentration) or (2) ultrafiltration-centrifugation (UltraF) as performed by Hazan-Halevy et al.1 For VSV-G- and HF-LV transductions, cells were harvested at 48 hours and total levels of reverse transcribed proviral DNA (total LTR/cell) were determined by quantitative polymerase chain reaction (see “Methods” in Frecha et al3 ). Freshly isolated, nonstimulated B cells of different B-CLL (C) and MZL (D) donors were transduced with 2 different preparations of H/F-LVs (MOI 5, HF-LV1 and HF-LV2, low-speed concentration) or with VSV-G-LVs (MOI 50, VSV-G-LV1, VSV-G-LV2, low-speed concentration) for 48 hours. Cells were washed subsequently and maintained in culture on MS5 stromal cells in RPMI supplemented with 10% fetal calf serum, IL-2, and IL-15 for 5 more days before FACS analysis was performed as in panel A. (E-F) The same transduction protocols as described for panels C and D were applied on B-CLL and MZL cells prestimulated for 48 hours with Staphylococcus aureus Cowen and IL-2 (10 ng/mL). Note that for non- and BCR-stimulated MZL-3 cells, transduction was performed with only the H/F-LV1 vector preparation. Similarly, prestimulated B-CLL-4 and B-CLL-10 cells were transduced with only H/F-LV1. FACS analysis was performed 2 days after transduction followed by 5 days of culture on MS5 cells to allow evaluation of stable transduction.
We speculate that the ultrafiltration resulted in vector concentration accompanied by strong protein concentration including GFP present in the viral supernatant. Incubation of B-CLLs or MZLs with ultrafiltration-concentrated LVs may lead to sticking of proteins (including GFP) to the cell surface, resulting in a green fluorescent signal. Note that we use low-speed concentration for our LVs, for which this phenomenon was not observed.3,6 Clearly, these results were similar for multiple donors: we transduced nonstimulated B cells of 12 different B-CLL donors (Figure 1C; and Frecha et al3 ) and 6 MZL donors (Figure 1D) with low-speed concentrated VSV-G-LVs (multiplicity of infection [MOI] 50) and H/F-LVs (MOI 5). Indeed, VSV-G-LVs do not permit an efficient transduction while H/F-LVs permit high transduction coinciding with signaling-lymphocyte-activating-molecule expression, one of the measles virus receptors (Figure 1C-D; and data not shown3,6 ). This poor gene transfer profile for VSV-G-LVs was also evident when the cells were prestimulated through the B-cell receptor (Figure 1E-F), whereas HF-LV transduction augmented significantly.3
In conclusion, HF-LVs are the candidates of choice for transducing B-CLLs or MZLs, and are highly superior to VSV-G-LVs.
Authorship
Acknowledgments: We thank Isabelle Dusanter for her input and discussion.
We acknowledge the support by the “Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales” (ANRS) and the European Community (FP7-HEALTH-2009-222878-PERSIST), the European Research Council (ERC-2008-AdG-233130-HEPCENT).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Els Verhoeyen or François-Loïc Cosset, EVIR Inserm U758, ENS de Lyon, 46 Allée d'Italie, 69364 Lyon Cedex 07, France; e-mail: els.verhoeyen@ens-lyon.fr or flcosset@ens-lyon.fr.
References
Author notes
C.L., C.F., F.-L.C., and E.V. contributed equally to this correspondence.