Migration of megakaryocytes (MKs) from the proliferative osteoblastic niche to the capillary-rich vascular niche is essential for proplatelet formation and platelet release. In this study, we explore the role of surface glycoprotein receptors and signaling proteins in regulating MK migration and platelet recovery after immune-induced thrombocytopenia. We show that spreading and migration of mouse primary bone marrow–derived MKs on a fibronectin matrix are abolished by the Src family kinases inhibitor PP1, the Syk kinase inhibitor R406 and the integrin αIIbβ3 antagonist lotrafiban. We also demonstrate that these responses are inhibited in primary phospholipase C γ2 (PLCγ2)–deficient MKs. Conversely, MK spreading and migration were unaltered in the absence of the collagen receptor, the glycoprotein VI–FcRγ-chain complex. We previously reported a correlation between a defect in MK migration and platelet recovery in the absence of platelet endothelial cell adhesion molecule-1 and the tyrosine phosphatase CD148. This correlation also holds for mice deficient in PLCγ2. This study identifies a model in which integrin signaling via Src family kinases and Syk kinase to PLCγ2 is required for MK spreading, migration, and platelet formation.
Introduction
Megakaryocytes (MKs) are the cellular precursor of platelets. They are formed in the proliferative osteoblastic niche of the bone marrow (BM) from hematopoietic progenitor cells. Mature MKs migrate to the vascular-rich niche where they bind to BM endothelial cells and generate proplatelets, which protrude into the bloodstream,1,2 with the final stage of platelet formation occurring in the blood.3 This series of events is regulated by a variety of chemokines and cytokines, as well as by adhesive interactions with extracellular matrix proteins.
Dynamic regulation of MK migration is critical for the ability of primary MKs to migrate to the vascular niche and promote proplatelet formation and platelet release. The chemokine, stromal cell–derived factor 1α (SDF1α), plays a vital role in the migration of MKs from the proliferative osteoblastic niche to the vascular niche through its receptor CXCR4.2 In addition, SDF1α promotes the interaction and transmigration of mature MKs through BM endothelial cells, resulting in augmentation of platelet release.4,5 The mechanisms that regulate migration of MKs toward a gradient of SDF1α are largely unknown.
Integrins play a central role in regulating cell migration.6 The integrin subunit αIIb (glycoprotein [GP]IIb), a marker for early hematopoietic progenitors,7 is highly expressed on the MK-platelet lineage as a complex with the β3 subunit (GPIIIa) to form the integrin αIIbβ3 (GPIIb-IIIa) complex.8 Fibronectin is a major component of BM extracellular matrix where it plays an important role in hematopoiesis.9,10 Fibronectin is distributed throughout the central region of the BM11 where hematopoietic cells are also found at high level.12 It has been proposed that fibronectin modulates migration and homing of hematopoietic cells to specific BM regions that it regulates maturation of progenitors and release of mature blood cells into the circulation. αIIbβ3 is the major receptor in MKs and platelets for fibronectin, although this also binds to α5β1 and αvβ3.13 However, αIIbβ3 is considered to be the most important of these due to its high levels of expression, which is approximately 2 orders of magnitude greater than that of the other 2 integrins.
Fibrillar collagen type I has also been described as an important component of the BM environment.11 The main receptors interacting directly with collagen are integrin α2β1 and GPVI.14 GPVI is restricted to platelets and MKs and is synthesized early during MK differentiation, but only become fully functional in mature MKs.15 Collagen is unable to support proplatelet formation and also prevents premature release of platelets within BM.16,–18
Src family kinases (SFKs) and Syk kinase play critical roles in inside-out activation of integrins by glycoprotein receptors such as the collagen receptor GPVI.19,–21 In addition, they have an important role in outside-in signals from integrins that mediate changes in cytoskeletal organization leading to cell spreading and motility.22 MKs and platelets contain high levels of several Src tyrosine kinases, including Fyn, Lyn and Src, as well as Syk kinase.23 Lyn has been reported to inhibit MK maturation,24,25 but its role, and that of other Src kinases and also Syk kinase, in mediating MK migration is not known.
We have previously reported critical roles for platelet endothelial cell adhesion molecule-1 (PECAM-1) and the protein tyrosine phosphatase CD148 in regulating MK migration on fibronectin and platelet recovery after immune-induced thrombocytopenia.26,27 Although the molecular explanation for the role of these 2 proteins in regulating migration is unclear, it is noteworthy that CD148 regulates Src kinase activity in platelets and that PECAM-1, through the nontransmembrane protein tyrosine phosphatases, Shp1 and Shp2, supports outside-in signaling through integrins in platelets and in other cells.27,–29 Conversely, PECAM-1 has also been shown to mediate weak inhibition of constitutive and agonist-induced signaling by immunoreceptor tyrosine-based activation motif (ITAM) receptors in platelets, and it is therefore unclear which of these actions, if any, underlie the role of the immunoglobulin immunoreceptor tyrosine-based inhibition motif (ITIM) receptor in supporting migration.30,,–33
In the present study, we provide evidence that SFKs, Syk kinase and PLCγ2, are critical components of αIIbβ3-mediated outside-in signaling in MKs and in the regulation of primary MK migration. Furthermore, we show that platelet recovery after induced thrombocytopenia is impaired by the absence of PLCγ2. The results, together with our previous observations, lead us to propose a model through which integrin αIIbβ3 and signaling proteins regulate MK migration. These observations also reveal new roles for platelet proteins at the level of the MK that have important implications for use of knockout mouse models.
Methods
Chemicals
Recombinant murine TPO, SCF, and SDF1α were purchased from PeproTech. Sheep anti–rat IgG Dynabeads, biotin-conjugated rat anti–mouse CD45R/B220, purified rat anti–mouse CD16/CD32, and fluorescein isothiocyanate (FITC)–conjugated anti–mouse CD41 were from BD Pharmingen. FITC-conjugated anti–mouse IgG and CXCR4 antibodies were from R&D Systems. Anti–mouse Ly-6G, biotin anti–mouse CD11b, and FITC-conjugated anti–mouse CD34 antibodies were from eBioscience. Anti–mouse CD49e antibody was from Serotec. Stempro medium and Dulbecco modified Eagle medium were from GIBCO/Invitrogen. Anti-mouse GPIbα antibody was from Emfret Analytics. Lotrafiban was a generous gift from GlaxoSmithKline. Anti-PLCγ2 (DN84) and anti-Syk (BR15) polyclonal antibodies were gifts from Dr Joseph Bolen (DNAX Research Institute, Palo Alto, CA). Bovine serum albumin (BSA; fatty acid–free) and ribonuclease A were purchased from Sigma-Aldrich. The Src family kinases (SFKs) inhibitor PP1 pyrazolopyrimidine was from Merck Biosciences. The Syk kinase inhibitor R406 was a gift from Dr David Simmons (Cellzome Ltd, Cambridge, United Kingdom). Bovine plasma fibronectin was purchased from Calbiochem. The anti-Src pan, anti-SFK activation loop phospho-Tyr-418 and anti-Src phospho-Tyr-529 antibodies were obtained from Biosource. The anti–phospho-myosin light chain (MLC; Thr18/Ser19) and anti-MLC antibodies were purchased from Cell Signaling Technology.
Mice
FcR γ-chain–deficient (γ-chain−/−) mice on a C57BL/6 background were kindly provided by Dr Takashi Saito (Chiba University Graduate School of Medicine, Chiba, Japan).34 Mice deficient in PLCγ2 on a C57BL/6 background were generated as described.35 Wild-type littermates were used as controls. All procedures were undertaken with United Kingdom Home Office approval in accordance with the Animals (Scientific Procedures) Act of 1986.
Preparation of mouse megakaryocytes and culture
Mature MKs from mouse BM were defined as the population of cells generated using the methodology previously described.26,36 In brief, BM cells were obtained from femora and tibiae of knockout mice and control littermates by flushing, and cells expressing one or more of the lineage-specific markers on their surface (CD16/CD32+, Gr1+, B220+, CD11b+) were depleted using immunomagnetic beads (sheep anti–rat IgG Dynabeads). The remaining population was cultured in 2.6% serum-supplemented Stempro medium with 2mM l-glutamine, penicillin/streptomycin, and 20 ng/mL murine stem cell factor (SCF) at 37°C under 5% CO2 for 2 days. Cells were then cultured for a further 4 days in the presence of 20 ng/mL SCF and 50 ng/mL of murine thrombopoietin (TPO). After 4 days of culture in the presence of TPO, the cell population was enriched in mature MKs using a 1.5%/3% BSA gradient under gravity (1g) for 45 minutes at room temperature as described.37
Megakaryocyte ploidy and flow cytometry
Expression levels of GPIIb and CXCR4 were measured by flow cytometry using specific antibodies. Polyploidy of mature MKs isolated by BSA gradient was analyzed after anti-GPIIb labeling and DNA staining with 0.01 mg/mL propidium iodide. DNA content was measured in GPIIb-positive cells. Samples were acquired using FACSCalibur flow cytometer and CellQuest software (Becton Dickinson) and analyzed using Summit Version 4.3 software (DAKO).
Cell migration assay
Chemotaxis was assessed using the Dunn chamber (Weber Scientific International) as described previously.26 To investigate the role of SFKs, Syk kinase, and integrins in migration, the Dunn chamber outer well was filled with the medium containing SDF1α (300 ng/mL) and the SFKs inhibitor PP1 (10μM), the Syk kinase inhibitor R406 (2μM) or the integrin antagonist lotrafiban (10μM). Time-lapse images were digitally captured every minute for 3 hours using a Zeiss 20×/1.40 NA plan-apochromat lens on a Zeiss Axiovert 200 inverted high-end microscope and a Hamamatsu Orca 285 cooled digital camera. Slidebook (3I, http://www.intelligent-imaging.com/) and ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/) software were used to acquire and process images.
Immune thrombocytopenia
Thrombocytopenia was induced in 2- to 4-month-old WT and KO mice by intraperitoneal injection of anti–mouse GPIbα antibody (2 μg/g of mouse weight), as previously described.26,27,38 Blood samples were collected before injection (time = 0) and then at 3, 48, 72, 96, 120, 144, and 172 hours after injection by tail bleeding. Platelet counts were measured using an ABX Pentra 60 Hematology Analyzer (Block Scientific Inc).
Megakaryocyte spreading
Coverslips were coated with fibronectin (20 μg/mL) or BSA (100 μg/mL) overnight at 4°C. After washing twice with PBS, the coverslips were blocked with denatured BSA (5 mg/mL) for 1 hour at room temperature, and washed twice with PBS before use in spreading experiments. Mature MKs were plated on fibronectin-coated surface for 3 hours at 37°C. Adherent MKs were fixed and permeabilized with Triton (0.1%) and actin fibers were stained with rhodamine-phalloidin.
Megakaryocyte biochemistry
To study tyrosine phosphorylation events in response to adhesion to fibronectin matrix, 6-well plates were coated with fibronectin (20 μg/mL) or BSA (100 μg/mL) overnight at 4°C and then blocked with denatured BSA (5 mg/mL) for 1 hour at room temperature. After BSA gradient, mature MKs were harvested 4 hours at 37°C and then were added to fibronectin or BSA coated dishes for 3 hours. MKs were either pretreated with DMSO (< 0.1%) or with inhibitors PP1 (10μM) or R406 (2μM) for 15 minutes at 37°C before plating. MKs adherent to fibronectin or in suspension over BSA were lysed in ice-cold immunoprecipitation buffer27 and lysates were subjected to immunoprecipitation assays. Samples were precleared for 30 minutes at 4°C with 20 μL protein A–Sepharose (50%, wt/vol). Precleared supernatants were incubated with 2 μL anti-PLCγ2 pAb (DN84) or 2 μL of anti-Syk Ab (BR15) and 20 μL of protein A Sepharose and were rotated overnight at 4°C. The Sepharose pellet was washed 3 times in lysis buffer before addition of Laemmli standard sample buffer. Precleared whole-cell lysates (WCLs) and immunoprecipitates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 4% to 12% gradient gels (Invitrogen Ltd) and immunoblotted with primary antibodies and horseradish peroxidise–conjugated secondary antibodies. Proteins were detected by enhanced chemiluminescence (GE Healthcare).
Statistical analysis
Experiments were performed a minimum of 3 times, and images shown are representative data from 1 experiment. Data are shown as mean plus or minus SEM. Statistical analysis was conducted using 2-tailed Student t test. P values of less than .05 were considered statistically significant.
Results
Src family kinases, Syk, and αIIbβ3 are required for MK migration
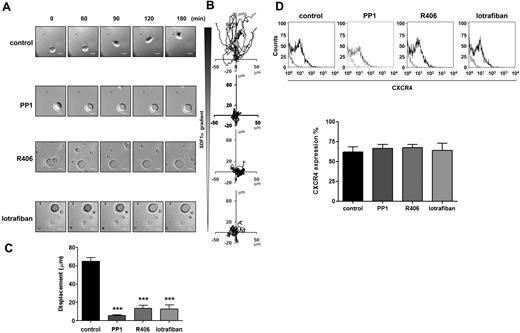
The role of SFKs and Syk kinase in MK migration on fibronectin toward a gradient of the major chemokine involved in MK migration, SDF1α,39 was investigated in a Dunn chamber assay using the SFKs and Syk inhibitors PP140 and R406,41 respectively. Migration was monitored as previously described.26,27 The concentrations of PP1 (10μM) and R406 (2μM) have been previously shown to cause maximal inhibition of Src and Syk kinases in platelets.22,42 In the presence of the vehicle dimethyl sulfoxide, MKs migrate in the direction of the SDF1α gradient as shown in Figure 1A-C. Migration toward SDF1α was completely inhibited by PP1 and R406, with minimal movement away from the starting location (Figure 1A-C; supplemental Videos 1 and 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Integrins play a critical role in migration of most cells and Src and Syk tyrosine kinases play a key role in outside-in integrin signaling.19,20 Migration of MKs on fibronectin was also abolished in the presence of the αIIbβ3 antagonist, lotrafiban43 (Figure 1A-C). Further, the level of the SDF1α chemokine receptor CXCR4 was unaffected by the treatment with the inhibitors for the duration of the experiment (Figure 1D). These results are consistent with a model in which migration of MKs on fibronectin matrix toward a SDF1α gradient is mediated through αIIbβ3-dependent activation of SFKs and Syk tyrosine kinase. However, they are also consistent with a model in which constitutive signaling by SFKs and Syk kinase could lie upstream of integrin activation.
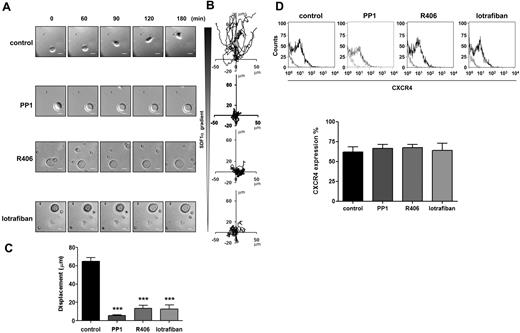
Role of SFKs, Syk kinase and integrins in MK migration toward a SDF1α gradient. Purified BM-derived mature MKs adherent on fibronectin (20 μg/mL)–coated coverslip were allowed to migrate toward a SDF1α gradient over 3 hours within the Dunn chamber as described in “Cell migration assay.” To investigate the role of SFKs, Syk kinase and integrins, the Dunn chamber outer well were filled with media containing SDF1α (300 ng/mL) and PP1 (10μM), R406 (2μM) or lotrafiban (10μM). (A) Representative differential interference contrast (DIC) images from 6 independent experiments of primary MKs exposed to a SDF1α gradient are shown (scale bar = 20μm). (B) The migration paths over 3 hours were traced. The intersection of the x- and y-axes was taken to be the starting point of each cell path, whereas the source of SDF1α was at the top. (C) The net translocation distance (displacement from the start to the end point) of each cell in the absence or presence of PP1, R406 and lotrafiban is represented. ***P < .005. See also supplemental Figure 1 videos 1 and 2. (D) CXCR4 surface expression of purified BM-derived mature MKs in the absence or presence of PP1, R406, or lotrafiban was analyzed by flow cytometry using a FITC-conjugated anti-CXCR4 antibody. Gray line indicates relevant control antibody; black line, FITC-conjugated anti-CXCR4 antibody. Representative profile and quantification of the percentage of cells expressing CXCR4 are from 3 independent experiments.
Role of SFKs, Syk kinase and integrins in MK migration toward a SDF1α gradient. Purified BM-derived mature MKs adherent on fibronectin (20 μg/mL)–coated coverslip were allowed to migrate toward a SDF1α gradient over 3 hours within the Dunn chamber as described in “Cell migration assay.” To investigate the role of SFKs, Syk kinase and integrins, the Dunn chamber outer well were filled with media containing SDF1α (300 ng/mL) and PP1 (10μM), R406 (2μM) or lotrafiban (10μM). (A) Representative differential interference contrast (DIC) images from 6 independent experiments of primary MKs exposed to a SDF1α gradient are shown (scale bar = 20μm). (B) The migration paths over 3 hours were traced. The intersection of the x- and y-axes was taken to be the starting point of each cell path, whereas the source of SDF1α was at the top. (C) The net translocation distance (displacement from the start to the end point) of each cell in the absence or presence of PP1, R406 and lotrafiban is represented. ***P < .005. See also supplemental Figure 1 videos 1 and 2. (D) CXCR4 surface expression of purified BM-derived mature MKs in the absence or presence of PP1, R406, or lotrafiban was analyzed by flow cytometry using a FITC-conjugated anti-CXCR4 antibody. Gray line indicates relevant control antibody; black line, FITC-conjugated anti-CXCR4 antibody. Representative profile and quantification of the percentage of cells expressing CXCR4 are from 3 independent experiments.
Phosphorylation of Src kinases, Syk, and PLCγ2 by integrin αIIbβ3
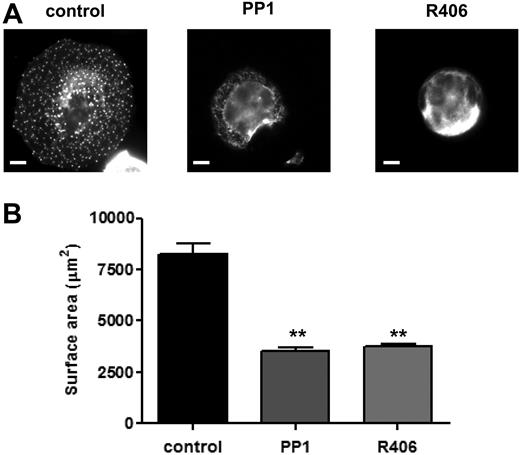
The role of SFKs and Syk kinase in MK spreading on fibronectin was investigated. MKs underwent marked spreading and formed prominent punctuate, actin-rich structures known as podosomes upon adhesion to a fibronectin surface, whereas they failed to spread on a BSA-coated surface (Figure 2 and data not shown). Spreading and podosomes formation were blocked in the presence of PP1 (10μM) and R406 (2μM), revealing a critical role for SFKs and Syk kinase, respectively, in these events (Figure 2).
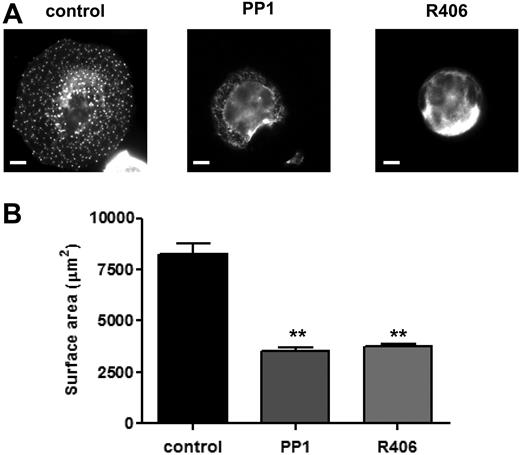
Role of SFKs and Syk kinase in MK spreading and cytoskeletal reorganization. Purified BM-derived mature MKs were incubated in the presence or absence of PP1 (10μM) or R406 (2μM) for 15 minutes at 37°C. MKs were plated on fibronectin-coated surface for 3 hours at 37°C. Adherent MKs were fixed, permeabilized, and actin fibers stained with rhodamine-phalloidin. Representative images (A) and surface area quantification (B) from 4 independent experiments are shown (scale bar = 20μm). **P < .01.
Role of SFKs and Syk kinase in MK spreading and cytoskeletal reorganization. Purified BM-derived mature MKs were incubated in the presence or absence of PP1 (10μM) or R406 (2μM) for 15 minutes at 37°C. MKs were plated on fibronectin-coated surface for 3 hours at 37°C. Adherent MKs were fixed, permeabilized, and actin fibers stained with rhodamine-phalloidin. Representative images (A) and surface area quantification (B) from 4 independent experiments are shown (scale bar = 20μm). **P < .01.
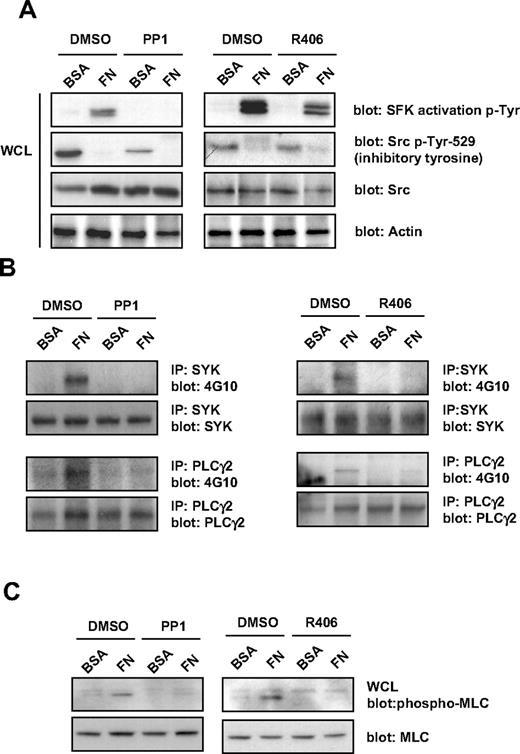
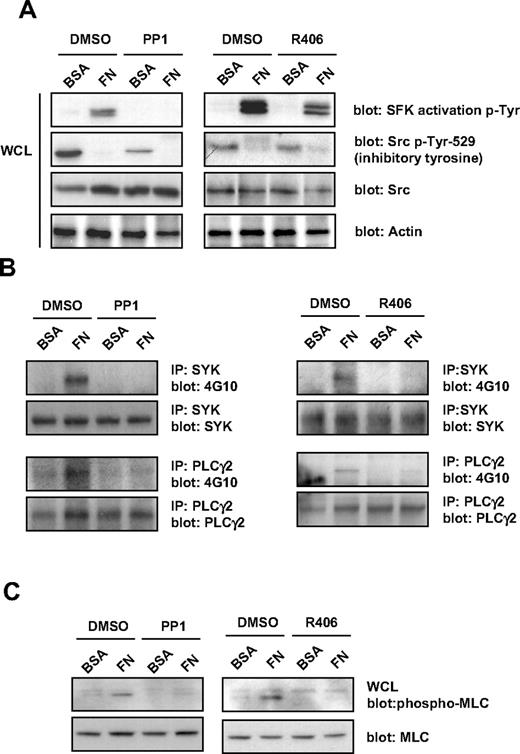
Protein phosphorylation studies were performed to further investigate the role of SFKs and Syk kinase in outside-in signaling by integrin αIIbβ3. Protein tyrosine phosphorylation was analyzed in MKs that had been exposed to fibronectin or BSA surfaces for 3 hours. Src kinases are phosphorylated at 2 distinct sites, Tyr-418 and Tyr-529. Autophosphorylation of Tyr-418 within the kinase activation loop is a marker of Src activation. Conversely, phosphorylation of Tyr-529 is a marker of Src suppression. Western blotting with a phosphotyrosine antibody, Tyr-418, that recognizes the active site of phosphorylation in all Src kinases, revealed marked phosphorylation in MKs incubated with fibronectin that was inhibited in the presence of PP1 (Figure 3A). Negligible phosphorylation at this site was observed in cells incubated with BSA (Figure 3A). In contrast, Western blotting with an antibody that recognizes the inhibitory site of phosphorylation in Src, Tyr-529, revealed significant phosphorylation in cells incubated with BSA, which was reduced in the presence of PP1, whereas there was minimal phosphorylation of this inhibitory site in cells incubated with fibronectin (Figure 3A). The partial reduction in phosphorylation of Tyr-529 suggests that, at this high concentration, PP1 also partially inhibits the Src inhibitory kinase, Csk. The further loss of phosphorylation of this site in the presence of fibronectin suggests activation of a phosphatase. These results demonstrate activation of Src kinases in MKs that had been allowed to adhere to fibronectin.
Effect of SFKs and Syk kinase inhibitors on integrin-induced tyrosine phosphorylation in MKs. Purified BM-derived mature MKs were preincubated for 15 minutes with PP1 (10μM) or R406 (2μM), plated on fibronectin (FN) or BSA-coated dish for 3 hours. (A) MKs were lysed and whole-cell lysates (WCLs) were analyzed by Western blot with SFK activation loop p-Tyr-418, Src inhibitory site p-Tyr-529, pan Src, and actin antibodies. Blots are representative of 3 independent experiments. (B) Syk and PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with an anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. Blots are representative of 3 independent experiments. (C) MKs were lysed and WCLs were analyzed by Western blot with MLC-P and MLC antibodies. Blots are representative of 3 independent experiments.
Effect of SFKs and Syk kinase inhibitors on integrin-induced tyrosine phosphorylation in MKs. Purified BM-derived mature MKs were preincubated for 15 minutes with PP1 (10μM) or R406 (2μM), plated on fibronectin (FN) or BSA-coated dish for 3 hours. (A) MKs were lysed and whole-cell lysates (WCLs) were analyzed by Western blot with SFK activation loop p-Tyr-418, Src inhibitory site p-Tyr-529, pan Src, and actin antibodies. Blots are representative of 3 independent experiments. (B) Syk and PLCγ2 were immunoprecipitated from equal amounts of WCLs and blotted with an anti-phosphotyrosine antibody. Membranes were subsequently stripped and reblotted with anti-Syk and anti-PLCγ2 antibodies. Blots are representative of 3 independent experiments. (C) MKs were lysed and WCLs were analyzed by Western blot with MLC-P and MLC antibodies. Blots are representative of 3 independent experiments.
Tyrosine phosphorylation of Syk, PLCγ2, and serine-threonine phosphorylation of MLC were also observed in MKs that had been exposed to fibronectin-coated but not BSA-coated dishes, and which was also blocked in the presence of PP1, demonstrating regulation downstream of SFKs (Figure 3B-C). In contrast, the Syk tyrosine kinase inhibitor, R406, only blocked tyrosine phosphorylation of SFKs on their activation tyrosine residue and had a minimal effect on phosphorylation at their inhibitory sites, but completely blocked tyrosine phosphorylation of Syk, PLCγ2, and MLC (Figure 3A-C).
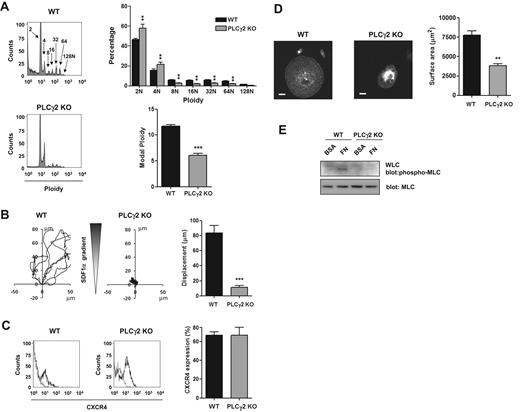
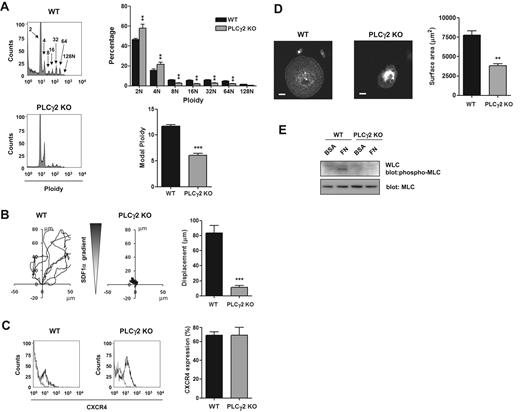
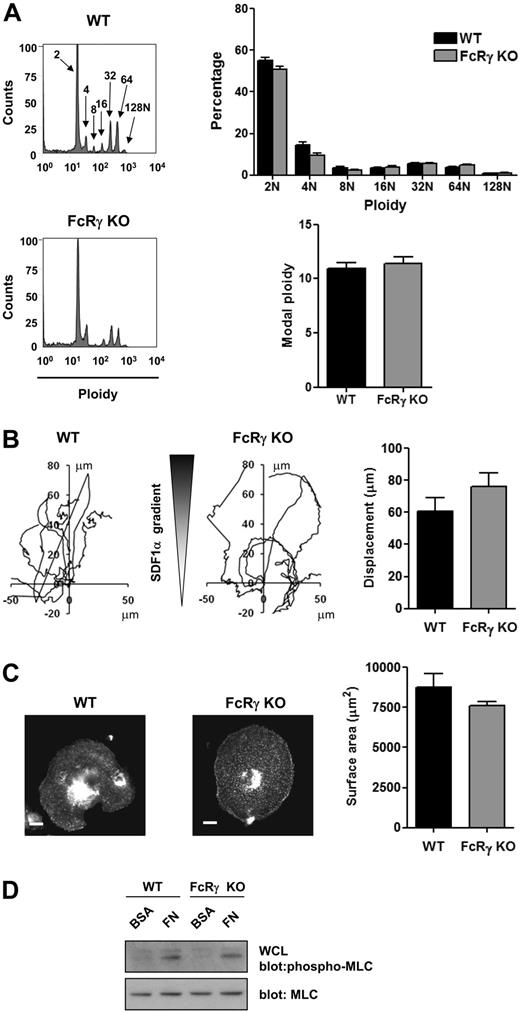
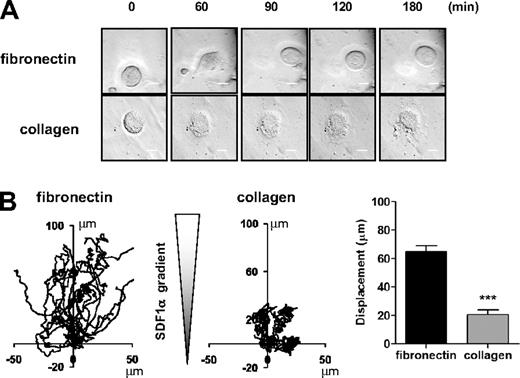
These data are consistent with the established pathway of signaling by αIIbβ3 through sequential activation of Src and Syk tyrosine kinases, leading to tyrosine phosphorylation of PLCγ2 and phosphorylation of MLC. To investigate this further, we used primary MKs derived from PLCγ2-deficient mice. PLCγ2-deficient mice exhibit a defect of MK development as shown by a partial block at the 2N and 4N stage of differentiation, a marked reduction in the percentage of cells with high ploidy levels and in the modal ploidy value (Figure 4A). MK migration toward a gradient of SDF1α was also impaired in the absence of PLCγ2 (Figure 4B), although the level of expression of the SDF1α receptor CXCR4 was not altered (Figure 4C). Spreading and phosphorylation of MLC were also abolished in the absence of PLCγ2 (Figure 4D-E). In contrast, all these processes were unaffected in FcRγ-chain–deficient mice (Figure 5A-D), showing a critical role for PLCγ2 and supporting a role for integrin αIIbβ3 outside-in signaling in regulating migration. In contrast, MKs underwent marked spreading but did not migrate on a collagen matrix in response to a SDF1α gradient (Figure 6A-B and supplemental Video 3).
Critical role of PLCγ2 in MK differentiation, migration, and spreading. (A) DNA ploidy was analyzed using flow cytometry by staining purified BM-derived mature MKs from WT and PLCγ2-deficient mice with propidium iodide and FITC-CD41 antibody. Representative profiles and quantification of the percentage of cells with differing levels of ploidy and the modal ploidy from 3 independent experiments are shown. **P < .01; ***P < .005. (B) Purified BM-derived mature MKs from wild-type (WT) and PLCγ2 knockout (PLCγ2 KO) were exposed to a SDF1α gradient over 3 hours within the Dunn chamber and the net translocation distance of each cell was measured *** P < .005. (C) CXCR4 surface expression of purified BM-derived mature MKs from WT and PLCγ2-deficient mice (PLCγ2 KO) was analyzed by flow cytometry. Gray line indicates relevant control antibody; black line, FITC-conjugated anti-CXCR4 antibody. Representative profiles and quantification of the percentage of cells expressing CXCR4 from 3 independent experiments are shown. (D) Purified BM-derived mature MKs from WT and PLCγ2-deficient mice (PLCγ2 KO) were plated on fibronectin-coated surface for 3 hours. Adherent MKs were fixed and permeabilized and actin fibers stained with rhodamine-phalloidin. Representative images (scale bar = 20μm) and surface area quantification from 4 independent experiments are shown. **P < .01. (E) Purified BM-derived mature MKs from WT and PLCγ2-deficient mice (PLCγ2 KO) were plated on fibronectin (FN) or maintained in suspension in a BSA-coated dish (BSA) for 3 hours. MKs were lysed and WCLs were analyzed by Western blot with MLC-P and MLC antibodies. Blots are representative of 3 independent experiments.
Critical role of PLCγ2 in MK differentiation, migration, and spreading. (A) DNA ploidy was analyzed using flow cytometry by staining purified BM-derived mature MKs from WT and PLCγ2-deficient mice with propidium iodide and FITC-CD41 antibody. Representative profiles and quantification of the percentage of cells with differing levels of ploidy and the modal ploidy from 3 independent experiments are shown. **P < .01; ***P < .005. (B) Purified BM-derived mature MKs from wild-type (WT) and PLCγ2 knockout (PLCγ2 KO) were exposed to a SDF1α gradient over 3 hours within the Dunn chamber and the net translocation distance of each cell was measured *** P < .005. (C) CXCR4 surface expression of purified BM-derived mature MKs from WT and PLCγ2-deficient mice (PLCγ2 KO) was analyzed by flow cytometry. Gray line indicates relevant control antibody; black line, FITC-conjugated anti-CXCR4 antibody. Representative profiles and quantification of the percentage of cells expressing CXCR4 from 3 independent experiments are shown. (D) Purified BM-derived mature MKs from WT and PLCγ2-deficient mice (PLCγ2 KO) were plated on fibronectin-coated surface for 3 hours. Adherent MKs were fixed and permeabilized and actin fibers stained with rhodamine-phalloidin. Representative images (scale bar = 20μm) and surface area quantification from 4 independent experiments are shown. **P < .01. (E) Purified BM-derived mature MKs from WT and PLCγ2-deficient mice (PLCγ2 KO) were plated on fibronectin (FN) or maintained in suspension in a BSA-coated dish (BSA) for 3 hours. MKs were lysed and WCLs were analyzed by Western blot with MLC-P and MLC antibodies. Blots are representative of 3 independent experiments.
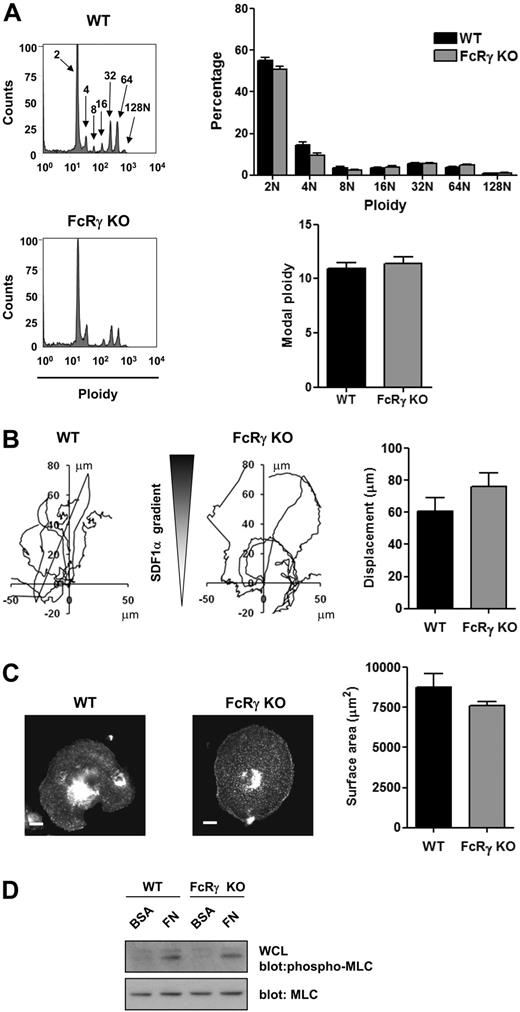
FcRγ-deficient mice demonstrate unchanged MK differentiation, migration, and spreading. (A) DNA ploidy distribution was analyzed by flow cytometry by staining purified BM-derived mature MKs from WT and FcRγ-deficient mice with propidium iodide and FITC-CD41 antibody. Representative profiles and quantification of the percentage of cells with differing levels of ploidy and the modal ploidy from 3 independent experiments are shown. (B) Purified BM-derived mature MKs from WT and FcRγ knockout (FcRγ KO) were exposed to a SDF1α gradient over 3 hours within the Dunn chamber and the net translocation distance of each cell was measured. (C) Purified BM-derived mature MKs from WT and FcRγ-deficient mice (FcRγ KO) were plated on fibronectin-coated surface for 3 hours. Adherent MKs were fixed, permeabilized and actin fibers stained with rhodamine-phalloidin. Representative images (scale bar = 20μm) and surface area quantification from 4 independent experiments are shown. (D) Purified BM-derived mature MKs from WT and FcRγ-deficient mice (FcRγ KO) were plated on fibronectin (FN) or maintained in suspension in a BSA-coated dish (BSA) for 3 hours. MKs were lysed and WCLs were analyzed by Western blot with MLC-P and MLC antibodies. Blots are representative of 3 independent experiments.
FcRγ-deficient mice demonstrate unchanged MK differentiation, migration, and spreading. (A) DNA ploidy distribution was analyzed by flow cytometry by staining purified BM-derived mature MKs from WT and FcRγ-deficient mice with propidium iodide and FITC-CD41 antibody. Representative profiles and quantification of the percentage of cells with differing levels of ploidy and the modal ploidy from 3 independent experiments are shown. (B) Purified BM-derived mature MKs from WT and FcRγ knockout (FcRγ KO) were exposed to a SDF1α gradient over 3 hours within the Dunn chamber and the net translocation distance of each cell was measured. (C) Purified BM-derived mature MKs from WT and FcRγ-deficient mice (FcRγ KO) were plated on fibronectin-coated surface for 3 hours. Adherent MKs were fixed, permeabilized and actin fibers stained with rhodamine-phalloidin. Representative images (scale bar = 20μm) and surface area quantification from 4 independent experiments are shown. (D) Purified BM-derived mature MKs from WT and FcRγ-deficient mice (FcRγ KO) were plated on fibronectin (FN) or maintained in suspension in a BSA-coated dish (BSA) for 3 hours. MKs were lysed and WCLs were analyzed by Western blot with MLC-P and MLC antibodies. Blots are representative of 3 independent experiments.
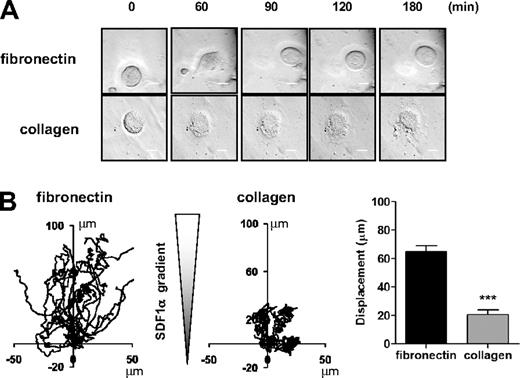
MK migration on collagen matrix is impaired. Purified BM-derived mature MKs adherent on fibronectin (20 μg/mL) or collagen (100 μg/mL)-coated coverslip were allowed to migrate toward a SDF1α gradient over 3 hours within the Dunn chamber. (A) Representative differential interference contrast (DIC) images from 6 independent experiments of primary MKs exposed to SDF1α gradient are shown (scale bar = 20μm). (B) The migration paths over 3 hours were traced and the net translocation distance of each cell on fibronectin and collagen matrix are represented. ***P < .005. See also supplemental Figure 6 video 3.
MK migration on collagen matrix is impaired. Purified BM-derived mature MKs adherent on fibronectin (20 μg/mL) or collagen (100 μg/mL)-coated coverslip were allowed to migrate toward a SDF1α gradient over 3 hours within the Dunn chamber. (A) Representative differential interference contrast (DIC) images from 6 independent experiments of primary MKs exposed to SDF1α gradient are shown (scale bar = 20μm). (B) The migration paths over 3 hours were traced and the net translocation distance of each cell on fibronectin and collagen matrix are represented. ***P < .005. See also supplemental Figure 6 video 3.
Role of signaling proteins in platelet formation
SDF1α-mediated MK migration from the endosteal niche to the vascular niche represents a critical step in the events that underlie proplatelet formation and platelet release into the blood circulation.2,5 The physiologic significance of the above signaling events in MK migration in vivo was investigated by measurement of platelet counts and platelet recovery after platelet depletion induced by an intraperitoneal injection of an anti-GPIbα antibody. These studies focused on the role of PLCγ2 and the collagen receptor complex, the GPVI-FcR γ-chain.
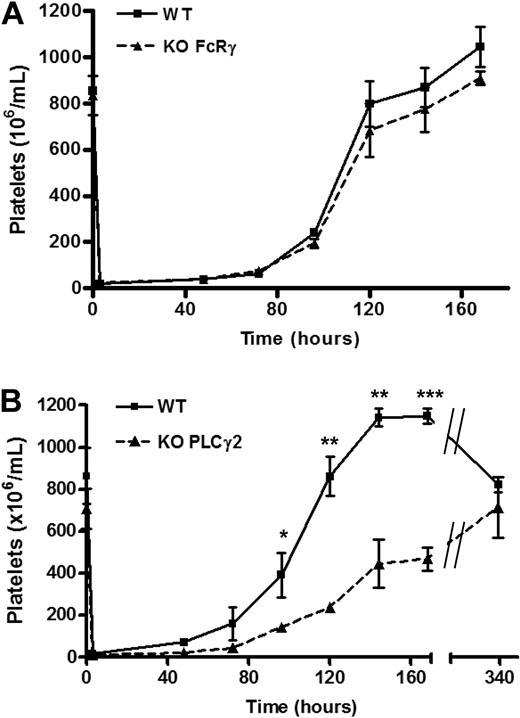
Mice deficient in PLCγ2 or the FcR γ-chain (which also fail to express GPVI)44 have a similar number of circulating platelets to those of litter-matched controls, consistent with previous studies.35 Furthermore, recovery of platelet counts after immune-induced thrombocytopenia in mice deficient in the FcR γ-chain is similar to that in litter-matched controls (Figure 7A). In contrast, a significant delay in recovery of the platelet counts after injection of GPIbα antibody was observed in PLCγ2-deficient mice that was still apparent after 7 days, but which had recovered by 2 weeks (Figure 7B). It is worth noting that in wild type mice, 7 days after immune-induced thrombocytopenia, the platelet counts was elevated relative to time 0 and only returns to the resting level after 2 weeks, suggesting overcompensation after acute depletion. Together, these results demonstrate a critical role for PLCγ2 in platelet formation after immune-induced thrombocytopenia but not in the regulation of the steady-state platelet count.
Platelet formation is regulated by PLCγ2 but not FcRγ-chain. Whole blood platelet counts from (A) WT (solid line) or FcRγ KO (dashed line) mice and (B) WT (solid line) or PLCγ2 KO (dashed line) were obtained before immune-induced thrombocytopenia (time point 0, n = 4), followed by an intraperitoneal injection of anti-mouse GPIbα antibody (2 μg/g of mouse weight). Platelet counts were then measured at indicated times (n = 4 for each time points). Error bars represent SEM. *P < .05; **P < .01; ***P < .005.
Platelet formation is regulated by PLCγ2 but not FcRγ-chain. Whole blood platelet counts from (A) WT (solid line) or FcRγ KO (dashed line) mice and (B) WT (solid line) or PLCγ2 KO (dashed line) were obtained before immune-induced thrombocytopenia (time point 0, n = 4), followed by an intraperitoneal injection of anti-mouse GPIbα antibody (2 μg/g of mouse weight). Platelet counts were then measured at indicated times (n = 4 for each time points). Error bars represent SEM. *P < .05; **P < .01; ***P < .005.
Discussion
As they mature, MKs migrate from the osteoblastic niche toward marrow sinusoids and protrude proplatelets through the vascular endothelium which release platelets in the vasculature. The BM environment contains extracellular matrix proteins and stromal cells that play a critical role in regulating MK migration and proplatelet formation. Interference with these processes can lead to production of platelets in BM. For example, premature formation of proplatelets and platelet formation in the BM have been reported in mice deficient in the actin-binding protein Wiskott-Aldrich syndrome protein (WASP) as a result of disturbance in both the negative regulation of proplatelet formation by collagen, which is mediated through integrin α2β1, and in MK migration induced by SDF1α.17 MyosinIIA, which is regulated by the Rho-Rho kinase pathway,45 also negatively regulates proplatelet formation, and mutations in the gene encoding myosinIIA have been shown to underlie the thrombocytopenia and presence of large platelets in the May-Hegglin (MHY9) group of disorders.46
In the case of the present study, we describe a novel integrin αIIbβ3-mediated signaling pathway that plays a critical role in regulating primary MK spreading and migration on fibronectin, and in platelet recovery after immune depletion of platelets. Integrin-mediated regulation of SFKs is vital for these processes as demonstrated by blockade of spreading and migration in the presence of the SFKs inhibitor PP1. Spreading and migration are also inhibited in the presence of the Syk kinase inhibitor R406 and in MKs derived from mice deficient in PLCγ2. Furthermore, platelet recovery after immune thrombocytopenia is reduced in mice deficient in PLCγ2, although the steady state platelet count is not altered, possibly due to the presence of PLCγ147 or other compensatory mechanisms. The defect in platelet recovery observed in PLCγ2-deficient mice is consistent with the impairment in MK migration, whereas in contrast, proplatelet formation is not altered in PLCγ2-deficient MKs.16 In addition to this pathway, it is possible that the negative role of SFKs in MK differentiation24,48 has also contributed to the reduction in migration, as fully differentiated MKs have a reduced migratory capacity.3,49
To better understand the mechanism by which SFKs and Syk kinase regulate fibronectin-induced MK migration, we examined phosphorylation of downstream signaling proteins targets. We found that integrin-mediated PLCγ2 phosphorylation is abolished by treatment with PP1 and R406, consistent with previous studies in mouse platelets that provided evidence for a critical role for SFKs and Syk kinase in integrin-mediated activation of PLCγ2.22,42,50 Moreover, we demonstrate that SFKs and Syk kinase regulate phosphorylation of MLC, which is likely to contribute to the changes in MK morphology and actin structure, both of which are required for MK migration.
Taken together, the present observations support a model in which the defect in platelet recovery observed after inhibition of SFKs, Syk kinase and PLCγ2 is due to impaired spreading and migration of MKs in BM, as a consequence of inhibited outside-in integrin signaling and the regulation of MLC kinase. Overlapping function between various integrins may explain the absence of a similar effect in the αIIb-knockout mouse.16
We have previously reported that MK spreading, migration and the rate of platelet recovery after immune-induced thrombocytopenia, are reduced in mice deficient in the protein tyrosine phosphatase CD148. This is believed to be due the role of CD148 in regulating global SFK activity in platelets.27 We have also reported a similar role for PECAM-1 in inhibiting MK migration and platelet recovery after immune thrombocytopenia, although in this case, MK spreading is increased.26 The latter is likely to be due to loss of inhibitory signals from the ITIM-containing protein which inhibit Src and Syk-dependent signaling.30 The corresponding increase in spreading, along with altered polarization of the chemokine SDF1α, is likely to contribute to the decrease in migration as a consequence of enhanced adhesion to matrix proteins in the BM.
In conclusion, this study defines a novel mechanism by which integrin αIIbβ3 promotes motility of primary BM-derived MKs through regulation of a SFKs-Syk-PLCγ2 signaling. Our findings therefore shed new light on the role of integrin-mediated signaling in MK migration and platelet formation. Further research using models that incorporate more of the complexity of the BM will help to elucidate key features of BM microenvironment in the regulation of MK migration in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Yan Zhao for technical assistance for platelet depletion. We also thank Drs Yotis Senis and Cedric Ghevaert for their constructive comments and help for the improvement of the manuscript. A.M. is a British Heart Foundation (BHF) Postdoctoral Research Fellow (PG/07/041/22 896). S. P. W. holds a BHF Chair (CH/03/003).
This work was supported by the BHF (PG/07/041).
Authorship
Contribution: A.M. designed and performed research, collected, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; S.G.T. discussed the results; T.S.D. and C.D.B. contributed analytical tools; and S.P.W. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexandra Mazharian, Centre for Cardiovascular Sciences, Institute of Biomedical Research, School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, Wolfson Dr, Edgbaston, Birmingham, B15 2TT, United Kingdom; e-mail: a.mazharian@bham.ac.uk.