Abstract
The anti-CD20 monoclonal antibody (mAb) rituximab has been used successfully for lymphoma therapy for more than 10 years. Although several direct mechanisms by which anti-CD20 mAbs act have been characterized in vitro, their specific role in clinical efficacy is still debated. Little is known about the possible antitumor immune response that they may induce in patients, despite clinical data suggesting a “vaccinal” effect. We show here that an initial treatment with anti-CD20 induces protection against human CD20-expressing tumor cells and allows immunocompetent mice to survive tumor challenge. This long-lasting protection requires the presence of the Fc portion of the anti-CD20 mAb and is achieved through the induction of a cellular immune response. Only CD4+ cells were needed at the beginning of the treatment, but both CD4+ and CD8+ cells were required after tumor challenge to achieve protection. Finally, we show that interleukin-2 treatment, given after tumor challenge, improves the overall survival rate, compared with that obtained by anti-CD20 treatment alone. These findings demonstrate that anti-CD20 mAbs exert therapeutic effects through the induction of an adaptive cellular immune response, aside from any direct mechanisms involving effectors from innate immunity.
Introduction
The anti-CD20 monoclonal antibody (mAb) rituximab has revolutionized the management of patients with follicular lymphoma (FL) and aggressive B-cell non-Hodgkin lymphoma (B-NHL) over the past decade.1-4 Other anti-CD20 mAbs, engineered to improve their effector functions, are currently being tested in clinical trials and may prove to be more effective therapeutic antibodies.5-8 This engineering has been based on studies that deciphered the mechanisms of action of these mAbs,9-11 principally programmed cell death induction, complement-dependent cytotoxicity, antibody-dependent cell cytotoxicity, and phagocytosis of mAb-opsonized target cells through receptors for the Fc portion of IgG (FcγRs).
In contrast, the long-term effects of anti-CD20 therapy on the host adaptive response have received little attention. Early in vitro studies indicated that rituximab promotes uptake of lymphoma cell-derived antigens by dendritic cells, thus generating tumor-specific cytotoxic T lymphocytes.12,13 A recent work showed that anti-CD20 mAb therapy synergizes with DC-based vaccination for long-lasting protection of mice with established CD20+ tumors.14 Two clinical observations suggest that rituximab therapy elicits a “vaccinal” effect that might prolong patient survival.9 First, time to progression in B-NHL patients who responded to rituximab and then relapsed (therefore, already a highly selected group) is longer after this second course of rituximab than after the first,15 possibly because the second course reduces the tumor load to a level that the patient's immune system can control by reactivating adaptive immune effectors and blocking immune suppression. Second, the clinical response rate in rituximab-responding B-NHL patients is higher when rituximab is administered as maintenance therapy.16 We hypothesize, therefore, that a longer period of tumor antigen exposure to immune effectors during maintenance therapy induces antitumor adaptive immune responses sufficient to increase the clinical response rate but not strong enough to ensure full protection from relapse.
Interestingly, the phase 2 clinical studies evaluating the combination of immunomodulatory cytokines with rituximab for treating B-NHL patients also suggest an anti-CD20 vaccinal effect.17,18 Combining interferon-α with rituximab in patients with indolent lymphoma seems to improve both the quality and duration of the clinical response.17 Similarly, combining granulocyte-macrophage colony-stimulating factor (GM-CSF) with rituximab for patients with relapsing or progressive FL produces high clinical response rates and a tolerable safety profile18 ; these authors suggest that the improved efficacy over rituximab alone is related to the increased number of cells expressing FcγRs (ie, monocytes, granulocytes, and dendritic cells).18 Mouse models have also demonstrated the role of FcγR-expressing cells in the clinical efficacy of IgG therapeutic antibodies. Studies of tumor-bearing mice show the importance of FcγRs in the DC-dependent induction of antitumor immune responses by immune complexes.19,20 Moreover, FcγR polymorphisms affect human clinical response to rituximab.21-23 This better clinical efficacy may be linked to enhanced antigen presentation by FcγR-expressing antigen-presenting cells.
A murine virus-induced tumor model has demonstrated the effect of mAb therapy on the host immune system by showing that the antimetastatic action of a mAb directed against the Friend leukemia virus envelope gp70 antigen depends on both the antibody Fc region and CD4+ cells.24 Furthermore, anti-gp70–treated mice produce antitumor antibodies that elicit tumor protection in naive recipient mice. The involvement of these mechanisms in the therapeutic activity of anti-CD20 mAb in vivo nonetheless remains unclear. We therefore investigated whether an anti-CD20 mAb induction regimen could trigger an adaptive antitumor immune response in immunocompetent mice. We show here that anti–human CD20 (huCD20) therapy provided long-lasting protection against huCD20+ tumor cells, allowing mice to survive to a subsequent tumor challenge. This protective Fc-dependent effect was achieved by inducing a cellular immune response that required the presence of both CD4+ and CD8+ cells, although only CD4+ cells were needed after the initial anti-huCD20 regimen. We also show that interleukin-2 (IL-2) therapy, administered after the second tumor injection, enhanced the overall survival rate of the mAb-treated mice after this injection.
Methods
Mice
Eight-week-old female C57Bl/6 mice were purchased from Charles River Laboratories. Eight-week-old female CD8 KO C57Bl/6 mice were obtained from Dr Jean-Charles Guéry (Inserm U563, Toulouse, France).
Cells
The mouse thymoma EL4 cells (EL4-wt) were purchased at ATCC. EL4 cells expressing human CD20 (EL4-huCD20) were kindly provided by Pr Hervé Watier (François Rabelais University, Tours, France). The CAT-13.6E12 hybridoma cell line was purchased from the DSMZ. This hybridoma cell line, established by Dr Martin Hadam (Kinderklinik-Medizinische Hochschule, Hanover, Germany), produces a typical type I anti-CD20 mAb. These cells were used to produce the anti-huCD20 CAT-13 IgG2a mAb. The stability and level of huCD20 expression on EL4-huCD20 cells were assessed by indirect immunofluorescence assays (FACSCalibur; BD Biosciences) with the CAT-13 mAb and phycoerythrin (PE)–conjugated F(ab′)2 goat anti–mouse (GAM) IgG (Fc-specific; Southern Biotechnology).
Antibody and F(ab′)2 fragment production and purification
CAT-13.6E12 culture supernatants were obtained by centrifugation at 270g and cleared by centrifugation at 4800g for 10 minutes at 4°C followed by 0.22-μm filtration (Millipore). CAT-13 mAb was purified by Protein-A affinity chromatography (rProtein A Sepharose fast flow; GE Healthcare). CAT-13 F(ab′)2 fragments were produced by pepsin digestion of the CAT-13 mAb, with immobilized pepsin (Pierce Chemical) according to the manufacturer's recommendations. Fragments containing Fc and trace amounts of undigested IgG were then removed by adsorption onto Protein A–coupled beads (rProtein A Sepharose fast flow; GE Healthcare). The purity of the CAT-13 F(ab′)2 fragments and the mAb was then assessed by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Invitrogen). The binding of CAT-13 mAb and F(ab′)2 fragments to EL4-huCD20 cells was measured by indirect immunofluorescence assays (FACSCalibur; BD Biosciences) with PE-conjugated F(ab′)2 GAM IgG (Fc-specific; Southern Biotechnology) and cyanine 3-conjugated F(ab′)2 GAM IgG (F(ab′)2-specific (Jackson ImmunoResearch).
Purified CAT-13 mAb was conjugated with Alexa Fluor 488 with the Alexa Fluor 488 monoclonal antibody labeling kit (Invitrogen).
Tumor immunotherapy
The C57Bl/6-EL4-huCD20 tumor model has been previously described25 and was slightly modified in the present study. Immunocompetent and CD8 KO C57Bl/6 mice were intravenously inoculated in the tail vein on day 0 with 5 × 105 EL4-huCD20 cells per mouse (in 200 μL of phosphate-buffered saline [PBS], pH 7.4). Surviving mice were intravenously challenged in the tail vein with either 5 × 105 EL4-wt or EL4-huCD20 cells (in 200 μL of PBS) on day 70. In one experiment, EL4-huCD20 cells were incubated for 24 hours (37°C, 5% CO2) with 5 μg/mL doxorubicin (DOX; Laboratoires Roger Bellon) before the intravenous inoculation on day 0.
CAT-13 mAb therapy was given as 5 intraperitoneal injections of 200 μg per mouse (in 200 μL of PBS, corresponding to 6.6μM) on days 1, 4, 7, 10, and 13. When CAT-13 F(ab′)2 fragments were tested, equivalent molar amounts were injected intraperitoneally (140 μg in 200 μL of PBS per mouse, corresponding to 6.7μM).
For CD4 depletion studies, anti-CD4–depleting mAb (GK1.5 clone, 200 μg per mouse in 200 μL of PBS; BioXcell) was injected intraperitoneally. Anti-CD4 mAb was injected either on day −1 (before initial tumor inoculation) or on day 69 (before tumor challenge) and repeated every 2 weeks.
For cotreatment studies with IL-2, human IL-2 (Roussel-Uclaf) was injected intraperitoneally (100 000 IU in 100 μL of PBS per mouse) either on days 1 and 4 (after initial tumor inoculation, concomitantly with CAT-13 mAb therapy) or days 71 and 74 (after tumor challenge).
Mice were killed when signs of malignancy appeared (eg, paralysis, body weight drop, prostration, and evident tumors). All animal studies were performed in compliance with institutional guidelines and the national charter on ethics in animal experiments (Charte nationale portant sur l'éthique de l'expérimentation animale) and were approved by the Ethics Committee in Animal Experiments Charles Darwin, Paris, France.
Serologic analysis
Blood samples were collected aseptically at different times. Serum was prepared in sterile conditions and kept frozen at −20°C until testing. The binding of PBS-diluted serum Ig to EL4-huCD20 or EL4-wt cells was measured by indirect immunofluorescence (FACSCalibur; BD Biosciences), with PE-conjugated F(ab′)2 GAM IgM (Fc-specific; Beckman Coulter), PE-conjugated F(ab′)2 GAM IgG (Fc-specific; Southern Biotechnology), or fluorescein isothiocyanate–conjugated F(ab′)2 GAM IgG subclasses (Fc-specific; IgG1, IgG2a, IgG2b, and IgG3; Southern Biotechnology).
Cytotoxicity assay
The anti–EL4-huCD20 cytotoxicity of spleen cells from naive and challenged CAT-13–treated mice (n = 3 in each group) was assessed by a standard 51Cr release assay: 5 × 105 EL4-huCD20 cells were labeled after incubation for 1 hour at 37°C with 37 μCi of 51Cr (PerkinElmer Life and Analytical Sciences). Spleen cells from challenged mice were isolated 3 days after the challenge. Cells were stimulated with mitomycin C (Sigma-Aldrich)–treated target EL4-huCD20 cells (5 × 104/well) at a 50:1 responder/stimulator ratio for 4 days in the presence of 100 IU/mL of human IL-2 (Roussel-Uclaf) to increase the frequency of tumor-specific cytotoxic T cells among whole spleen cells. In vitro–stimulated cells were then mixed with 51Cr-labeled EL4-huCD20 cells (5 × 103/well at effector/target ratios of 50:1, 100:1, and 200:1) and incubated for 4 hours at 37°C. The percentage of specific lysis was calculated with this formula: [(cpmexp − cpmmin)/(cpmmax − cpmmin)] × 100, wherein cpm indicates counts per minute; exp, experimental; min, spontaneous release; and max, maximum release.
Adoptive transfer experiments
Serum for the passive transfer experiment was collected in sterile conditions from untreated mice 20 days after tumor inoculation and from surviving CAT-13–treated mice 20 days after tumor challenge (day 90). On day −1, pooled serum samples were intravenously (thus passively) transferred from either untreated mice or surviving CAT-13–treated mice into the tail vein of naive recipient mice (200 μL of undiluted serum per mouse). Recipient mice and naive control mice were then inoculated intravenously with 5 × 105 EL4-huCD20 cells (in 200 μL of PBS per mouse) on day 0.
For the spleen cell adoptive transfer experiment, surviving CAT-13–treated mice were killed on day 90, 20 days after tumor challenge. Splenocytes were intravenously injected into the tail vein of naive recipient mice on day −1 (5 × 107 spleen cells in 200 μL of PBS per mouse). Recipient mice and naive control mice were then inoculated intravenously with 5 × 105 EL4-huCD20 cells (in 200 μL of PBS per mouse) on day 0.
Phenotypic analysis by direct immunofluorescence
Spleens from naive mice, untreated mice (20 days after tumor inoculation), and surviving CAT-13–treated mice (on day 90, 20 days after tumor challenge) were removed in sterile conditions. Spleen cells were incubated for 30 minutes at 4°C with 10 μg/mL rat anti–mouse (RAM) FcγR 2.4G2 mAb. Mouse CD3, CD4, and CD8 were visualized by incubation with PE-conjugated RAM CD3, PE-Texas red–conjugated RAM CD4, and Alexa Fluor 700–conjugated RAM CD8 mAbs (BD Biosciences PharMingen, Invitrogen, and eBioscience, respectively) for 30 minutes at 4°C. Isotype-matched mAbs were used as staining controls. Spleen cells were then washed in PBS containing 2% fetal calf serum and 2mM EDTA and fixed in PBS containing 0.5% formaldehyde. Staining was analyzed with the LSR II cytometer (BD Biosciences).
CD25 expression on EL4-huCD20 cells was assessed by direct immunofluorescence. Cells were isolated from tumor-infiltrated lymph nodes of tumor-bearing mice on day 35, incubated in vitro for 2 days in culture medium, and then overnight in the presence of human IL-2 (50, 500, and 5000 IU/mL). Direct immunofluorescence was performed with allophycocyanin-conjugated RAM CD25 (BD Biosciences PharMingen) and Alexa Fluor 488–conjugated CAT-13 mAb, which allows the detection of EL4-huCD20 cells. Staining was analyzed with the FACSCalibur (BD Biosciences).
Statistical analysis
The Kruskal-Wallis test was used to determine the significance of differences between sample means. Comparative survival was analyzed with the log-rank statistical test, selected to take into account both survival over the follow-up period and time-to-event (death or sacrifice). For statistical analyses, we used the StatView Version 5.0 software package for Windows (SAS Institute). Data are mean plus or minus SD when indicated.
Results
Anti-huCD20 mAb therapy after tumor inoculation led to long-lasting survival, including after a subsequent tumor challenge
To determine whether initial anti-huCD20 mAb therapy induces an antitumor response that can protect mice after tumor challenge, we injected 5 × 105 EL4-huCD20 cells intravenously into immunocompetent C57Bl/6 mice25 on day 0 and treated them with mouse IgG2a anti-huCD20 mAb CAT-13 (5 200-μg intraperitoneal injections on days 1, 4, 7, 10, and 13). The 5 × 200 μg induction regimen produced the highest overall survival rate. Figure 1 depicts a representative experiment. Approximately 80% of the CAT-13–treated mice survived to day 70 after the initial tumor injection (Figure 1A; P < .001). Overall survival from 7 independent experiments averaged approximately 60% (Table 1). All untreated mice died before day 50 (Figure 1A).25
Anti-huCD20 mAb therapy leads to long-lasting survival after tumor challenge in vivo. (A) Mice were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells (n = 25) and were divided into 2 groups. The first group (□, n = 8) was left untreated. Mice from the second group (■, n = 17) received CAT-13 mAb therapy (5 × 200 μg intraperitoneal injections on days 1, 4, 7, 10, and 13). *P < .001. Black arrows indicate CAT-13 mAb injections. (B) Surviving CAT-13–treated mice were challenged intravenously with either 5 × 105 EL4-wt cells (●, n = 7) or EL4-huCD20 cells (■, n = 7). Naive mice injected with either 5 × 105 EL4-wt (○, n = 7) or EL4-huCD20 cells (□, n = 7) were used as controls. **P < .001. Statistical comparisons were performed with the log-rank test.
Anti-huCD20 mAb therapy leads to long-lasting survival after tumor challenge in vivo. (A) Mice were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells (n = 25) and were divided into 2 groups. The first group (□, n = 8) was left untreated. Mice from the second group (■, n = 17) received CAT-13 mAb therapy (5 × 200 μg intraperitoneal injections on days 1, 4, 7, 10, and 13). *P < .001. Black arrows indicate CAT-13 mAb injections. (B) Surviving CAT-13–treated mice were challenged intravenously with either 5 × 105 EL4-wt cells (●, n = 7) or EL4-huCD20 cells (■, n = 7). Naive mice injected with either 5 × 105 EL4-wt (○, n = 7) or EL4-huCD20 cells (□, n = 7) were used as controls. **P < .001. Statistical comparisons were performed with the log-rank test.
Surviving CAT-13–treated mice were intravenously challenged on day 70 by either 5 × 105 EL4-wt or EL4-huCD20 cells, without any further mAb therapy. Approximately 70% of the surviving CAT-13–treated mice challenged with EL4-huCD20 cells were still alive at least 70 days after this challenge (ie, on day 140; Figure 1B; P < .001). In contrast, all surviving CAT-13–treated mice challenged with EL4-wt cells died within 25 days. Survival through day 140 of CAT-13–treated mice injected with EL4-huCD20 cells in 7 independent experiments averaged approximately 40% (Table 1).
The anti-huCD20 induction regimen therefore allowed the long-term survival of mice after injection of huCD20+ tumor cells. This protection allowed the mice to survive a subsequent tumor challenge with huCD20+ but not huCD20− tumor cells. The protection induced was thus huCD20-specific.
Xeno-immunization did not produce long-term antitumor protection, which required intact CAT-13 mAb
Recent studies have shown that dying tumor cells can intrinsically induce an antitumor immune response.26,27 Moreover, the presence of a human CD20 molecule on EL4 cells may trigger xeno-immunization that protects the surviving CAT-13–treated mice once they are challenged with these cells. We therefore examined whether the long-lasting antitumor protection was strictly CAT-13–dependent and not solely the result of the immunogenicity of dying EL4-huCD20 tumor cells. Mice were injected intravenously with 5 × 105 DOX-treated dying EL4-huCD20 cells on day 0. All animals survived with no sign of malignancy (Figure 2A). Surviving mice were then challenged with live 5 × 105 EL4-huCD20 cells on day 70. These mice died as rapidly as the untreated control mice (Figure 2B). This result suggests that injection of DOX-treated dying EL4-huCD20 cells did not trigger protective xeno-immunization.
Xeno-immunization does not produce long-term antitumor protection, which requires intact CAT-13 mAb in vivo. (A) Mice were intravenously injected on day 0 with either 5 × 105 DOX-treated EL4-huCD20 cells (×, n = 8) or 5 × 105 EL4-huCD20 cells (n = 14). The latter mice were divided into 2 groups. The first group was left untreated (□, n = 6) and the second received CAT-13 mAb therapy (■, n = 8). *P < .001. (B) Surviving CAT-13–treated mice (■, n = 6) and mice previously injected with DOX-treated EL4-huCD20 cells (×, n = 8) were challenged intravenously with 5 × 105 EL4-huCD20 cells. Control naive mice were injected intravenously with 5 × 105 EL4-huCD20 cells (□, n = 6). **P = .03. (C) Mice were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells (n = 18) and divided into 3 groups. The first group was left untreated (□, n = 6). The second group received the CAT-13 mAb therapy (■, n = 6). The third group received CAT-13 F(ab′)2 therapy (5 × 150 μg intraperitoneally on days 1, 4, 7, 10, and 13; ▴, n = 6). ***P < .001. Black arrows indicate CAT-13 mAb and CAT-13 F(ab′)2 fragment injections. Statistical comparisons were performed with the log-rank test.
Xeno-immunization does not produce long-term antitumor protection, which requires intact CAT-13 mAb in vivo. (A) Mice were intravenously injected on day 0 with either 5 × 105 DOX-treated EL4-huCD20 cells (×, n = 8) or 5 × 105 EL4-huCD20 cells (n = 14). The latter mice were divided into 2 groups. The first group was left untreated (□, n = 6) and the second received CAT-13 mAb therapy (■, n = 8). *P < .001. (B) Surviving CAT-13–treated mice (■, n = 6) and mice previously injected with DOX-treated EL4-huCD20 cells (×, n = 8) were challenged intravenously with 5 × 105 EL4-huCD20 cells. Control naive mice were injected intravenously with 5 × 105 EL4-huCD20 cells (□, n = 6). **P = .03. (C) Mice were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells (n = 18) and divided into 3 groups. The first group was left untreated (□, n = 6). The second group received the CAT-13 mAb therapy (■, n = 6). The third group received CAT-13 F(ab′)2 therapy (5 × 150 μg intraperitoneally on days 1, 4, 7, 10, and 13; ▴, n = 6). ***P < .001. Black arrows indicate CAT-13 mAb and CAT-13 F(ab′)2 fragment injections. Statistical comparisons were performed with the log-rank test.
Because the CAT-13 mAb therapy, and not the intrinsic immunogenicity of dying tumor cells, appears critical for the induction of long-term antitumor protection, we tested whether the CAT-13 Fc portion was required for this protection. After intravenous tumor inoculation on day 0, mice received 5 intraperitoneal injections of CAT-13 F(ab′)2 fragments. As shown in Figure 2C, all F(ab′)2-treated mice died before day 35, as the untreated mice did, thereby indicating that the Fc portion of the CAT-13 antibody is required for achieving a therapeutic effect.
Taken together, these results demonstrate that the therapeutic efficacy of the CAT-13 induction regimen requires intact IgG mAb and that EL4-huCD20 cells alone do not trigger a protective antitumor response.
Endogenous antibody response against tumor cells
We then evaluated whether CAT-13 mAb therapy could lead to long-lasting antitumor protection by inducing an endogenous antibody response against tumor cells. First, we used indirect immunofluorescence to analyze the presence of IgM and IgG directed against EL4-wt and EL4-huCD20 cells in the sera of CAT-13–treated mice on days 15, 30, 45, 70, 85, and 100. Of note, at all time points, the sera of CAT-13–treated mice showed only faint IgM binding to both EL4-wt (data not shown) and EL4-huCD20 cells (Figure 3A top panels). Similarly, only very weak IgG binding to EL4-wt cells was detected (data not shown). In contrast, IgG binding to EL4-huCD20 cells was easily detected on days 15, 30, and 45 (Figure 3A middle panels) but decreased over time. Interestingly, the lack of any increase in the antitumor IgG titer after the EL4-huCD20 tumor challenge on day 70 suggests that the IgG binding detected on days 15, 30, and 45 was mostly the result of the presence of CAT-13 IgG2a in the tested sera. To test this hypothesis, we analyzed the subclass specificity of IgG bound to EL4-huCD20 cells. No IgG1, IgG2b, or IgG3 binding to EL4-huCD20 cells could be detected (data not shown). In contrast, marked IgG2a binding to EL4-huCD20 cells was observed on days 15, 30, and 45 (Figure 3A bottom panels) and decreased thereafter. It was notably weak on days 85 and 100 (ie, 15 and 30 days after tumor challenge). Taken together, these results demonstrate the absence of any potent IgM and IgG response against EL4-huCD20 cells in CAT-13–treated mice. For additional confirmation of the absence of any antitumor antibody response that could be responsible for the protective effect, we pooled sera from CAT-13–treated surviving mice and used it in a passive immunity transfer experiment (Figure 3B). Recipient mice were intravenously injected in the tail vein on day −1 with 200 μL of pooled sera from either untreated (day 20 sera) or surviving CAT-13–treated mice (day 90 sera; ie, 20 days after the tumor challenge). Recipient mice were then intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells. The death of all the animals before day 35 showed that the CAT-13–induced long-term antitumor protection could not be adoptively transferred to naive recipients by a single injection of sera from surviving CAT-13–treated mice.
Analysis of the endogenous antibody response against tumor cells and passive immunity transfer in vivo. (A) IgM, IgG, and IgG2a binding to EL4-huCD20 cells was analyzed by indirect immunofluorescence assay with sera from CAT-13–treated and naive animals. Mean fluorescence intensities (MFI) are shown. Black arrows indicate the time of tumor challenge (day 70). (B) Naive recipient mice were intravenously injected on day −1 with either pooled sera from untreated mice (sera from day 20 after intravenous injection of EL4-huCD20 cells; ◇, n = 6) or pooled sera from surviving CAT-13–treated mice (sera from day 90, 20 days after intravenous tumor challenge; ♦ n = 6). Recipient mice subsequently had intravenous injections on day 0 of 5 × 105 EL4-huCD20 cells. Untreated mice (□, n = 6) were used as controls (log-rank analysis, not significant).
Analysis of the endogenous antibody response against tumor cells and passive immunity transfer in vivo. (A) IgM, IgG, and IgG2a binding to EL4-huCD20 cells was analyzed by indirect immunofluorescence assay with sera from CAT-13–treated and naive animals. Mean fluorescence intensities (MFI) are shown. Black arrows indicate the time of tumor challenge (day 70). (B) Naive recipient mice were intravenously injected on day −1 with either pooled sera from untreated mice (sera from day 20 after intravenous injection of EL4-huCD20 cells; ◇, n = 6) or pooled sera from surviving CAT-13–treated mice (sera from day 90, 20 days after intravenous tumor challenge; ♦ n = 6). Recipient mice subsequently had intravenous injections on day 0 of 5 × 105 EL4-huCD20 cells. Untreated mice (□, n = 6) were used as controls (log-rank analysis, not significant).
Together, these results indicate that the long-term antitumor protection induced by CAT-13 mAb therapy was not the result of the generation of a potent endogenous antibody response directed against the EL4-huCD20 cells.
CAT-13–induced antitumor protection was mediated through the triggering of a cellular immune response
We sought to determine whether the long-lasting CAT-13–induced antitumor protection involves cellular immune effectors. First, we examined whether spleen cells from CAT-13–treated animals exerted any anti–EL4-huCD20 activity in an in vitro cytotoxicity assay. Spleen cells from challenged mice were isolated 3 days after the tumor challenge (day 73), stimulated with mitomycin C–treated target EL4-huCD20 cells for 4 days in the presence of IL-2, and then incubated with 51Cr-labeled EL4-huCD20 cells. Figure 4A shows that stimulated spleen cells from CAT-13–treated challenged mice killed the target EL4-huCD20 cells, a finding that demonstrates that these animal spleens contained antitumor immune effectors. Second, we used splenocytes from surviving CAT-13–treated mice to test adoptive immunity transfer into naive recipients on day 90. Figure 4B shows that the intravenous injection of 5 × 107 spleen cells from surviving mice 24 hours before the intravenous injection of EL4-huCD20 tumor cells is sufficient enough to protect recipients from death (P = .03). That is, the long-lasting CAT-13–induced antitumor protection can be adoptively transferred into naive mice through spleen cells.
Spleen cells are involved in the CAT-13–induced antitumor protection. (A) Spleen cells from naive (□, n = 3) or CAT-13–treated challenged (day 73) mice (■, n = 3) were stimulated with mitomycin C-treated target EL4-huCD20 cells at a 50:1 responder/stimulator ratio for 4 days in the presence of IL-2. Stimulated cells were then mixed with 51Cr-labeled EL4-huCD20 cells at effector/target ratios of 50:1, 100:1, and 200:1. Effector values correspond to the number of CD3+ T cells. (B) A total of 5 × 107 spleen cells from surviving CAT-13–treated mice, isolated 20 days after tumor challenge, were intravenously injected into naive recipient mice on day −1 (♦ n = 5). These mice were subsequently intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells, as were control naive mice (□, n = 5; log-rank analysis, *P = .03). (C) Spleen cells from untreated or surviving CAT-13–treated mice were analyzed by immunofluorescence 20 days after tumor injection (untreated animals) or tumor challenge (surviving CAT-13–treated animals). Spleen cells from naive mice were also tested. The CD4+/CD8+ T-cell ratio was calculated (top panel; Kruskal-Wallis analysis, †P = .01), and the number of CD4+ and CD8+ cells among 3 × 105 CD3+ spleen cells was determined for each mouse (bottom panel; Kruskal-Wallis analysis, ††P = .008).
Spleen cells are involved in the CAT-13–induced antitumor protection. (A) Spleen cells from naive (□, n = 3) or CAT-13–treated challenged (day 73) mice (■, n = 3) were stimulated with mitomycin C-treated target EL4-huCD20 cells at a 50:1 responder/stimulator ratio for 4 days in the presence of IL-2. Stimulated cells were then mixed with 51Cr-labeled EL4-huCD20 cells at effector/target ratios of 50:1, 100:1, and 200:1. Effector values correspond to the number of CD3+ T cells. (B) A total of 5 × 107 spleen cells from surviving CAT-13–treated mice, isolated 20 days after tumor challenge, were intravenously injected into naive recipient mice on day −1 (♦ n = 5). These mice were subsequently intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells, as were control naive mice (□, n = 5; log-rank analysis, *P = .03). (C) Spleen cells from untreated or surviving CAT-13–treated mice were analyzed by immunofluorescence 20 days after tumor injection (untreated animals) or tumor challenge (surviving CAT-13–treated animals). Spleen cells from naive mice were also tested. The CD4+/CD8+ T-cell ratio was calculated (top panel; Kruskal-Wallis analysis, †P = .01), and the number of CD4+ and CD8+ cells among 3 × 105 CD3+ spleen cells was determined for each mouse (bottom panel; Kruskal-Wallis analysis, ††P = .008).
Because this protection is a durable phenomenon probably related to the generation of a cellular, but not humoral, antitumor immune response, we next evaluated the involvement of T cells. In a first set of experiments, we analyzed by flow cytometry the CD4+ and CD8+ T-cell subsets in the spleen and calculated the CD4+/CD8+ T-cell ratio among CD3+ cells (Figure 4C). Untreated mice injected with EL4-huCD20 cells and surviving CAT-13–treated mice challenged with a second injection of EL4-huCD20 cells were analyzed 20 days after the tumor cell challenge (that is, day 20 for the untreated mice, day 90 for the surviving treated mice). Naive C57Bl/6 mice were used as controls. The CD4+/CD8+ T-cell ratio was markedly lower 20 days after EL4-huCD20 cell injection in untreated mice than in both the naive and surviving CAT-13–treated mice (Figure 4C top histograms; P = .01). The lower CD4+/CD8+ T-cell ratio in untreated mice paralleled their markedly lower absolute number of CD3+CD4+ but not of CD3+CD8+ cells (Figure 4C bottom histograms; P = .008). Thus, the similarity in both splenic T-cell counts and CD4+/CD8+ T-cell ratios in naive and surviving CAT-13–treated mice and their differences from those of untreated tumor-bearing animals suggest that antibody treatment affects the splenic CD4+ T-cell subset.
To explore the role of CD4+ cells in antitumor protection, we used a depleting anti-CD4 mAb (GK1.5) to assess the effect of CD4+ cell depletion in C57Bl/6 mice at different times. In the first experiment, depletion began on day −1, before the EL4-huCD20 cell injection on day 0 and subsequent CAT-13 mAb therapy from days 1 to 13. Figure 5A shows that the therapeutic efficacy of the CAT-13 induction regimen was dramatically impaired when CD4+ cells were depleted before therapy began. Only 16% of CD4-depleted mice (2 of 12) survived on day 70 versus 58% (7 of 12) of CD4+ mice (P = .01). This result indicates that CD4+ cells are needed at the beginning of mAb therapy to achieve long-term survival. In a second experiment, depletion began on day 69, the day before the tumor challenge on day 70. All depleted mice died after this tumor challenge, and the mortality observed was similar to that of untreated control mice (Figure 5B). This experiment demonstrates that CD4+ cells are also involved in the survival of mice after tumor challenge.
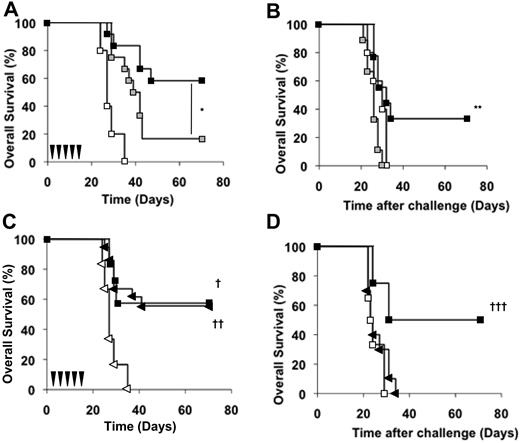
CD4+ and CD8+ cells are required for the CAT-13–induced antitumor protection in vivo. (A) Naive mice were depleted of CD4+ cells by intraperitoneal injections of the anti-CD4 mAb GK1.5 starting on day −1. Both depleted ( , n = 12) and nondepleted control mice (■, n = 12) were then injected with 5 × 105 EL4-huCD20 cells on day 0 and then received CAT-13 mAb therapy. Untreated mice (□, n = 6) were also used as controls (log-rank analysis, *P = .01). (B) Surviving CAT-13–treated mice received intraperitoneal injections of GK1.5 mAb starting on day 69 (
, n = 12) and nondepleted control mice (■, n = 12) were then injected with 5 × 105 EL4-huCD20 cells on day 0 and then received CAT-13 mAb therapy. Untreated mice (□, n = 6) were also used as controls (log-rank analysis, *P = .01). (B) Surviving CAT-13–treated mice received intraperitoneal injections of GK1.5 mAb starting on day 69 ( , n = 9) or not (■, n = 9) and were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 5) were used as controls (log-rank analysis, **P = .005). (C) Wild-type (n = 7) and CD8 KO C57Bl/6 mice (n = 18) were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells. Wild-type animals received the CAT-13 mAb therapy (□, n = 7). CD8 KO mice were divided into 2 groups. The first group was left untreated and used as a control (▷, n = 6). The second group of CD8 KO mice received the CAT-13 mAb therapy (◀, n = 18; log-rank analysis, †P = .01; ††P < .001). (D) Both wt (■, n = 4) and CD8 KO (◀, n = 10) surviving CAT-13–treated animals were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 6) were used as controls (log-rank analysis, †††P = .02). Black arrows indicate CAT-13 mAb injections.
, n = 9) or not (■, n = 9) and were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 5) were used as controls (log-rank analysis, **P = .005). (C) Wild-type (n = 7) and CD8 KO C57Bl/6 mice (n = 18) were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells. Wild-type animals received the CAT-13 mAb therapy (□, n = 7). CD8 KO mice were divided into 2 groups. The first group was left untreated and used as a control (▷, n = 6). The second group of CD8 KO mice received the CAT-13 mAb therapy (◀, n = 18; log-rank analysis, †P = .01; ††P < .001). (D) Both wt (■, n = 4) and CD8 KO (◀, n = 10) surviving CAT-13–treated animals were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 6) were used as controls (log-rank analysis, †††P = .02). Black arrows indicate CAT-13 mAb injections.
CD4+ and CD8+ cells are required for the CAT-13–induced antitumor protection in vivo. (A) Naive mice were depleted of CD4+ cells by intraperitoneal injections of the anti-CD4 mAb GK1.5 starting on day −1. Both depleted ( , n = 12) and nondepleted control mice (■, n = 12) were then injected with 5 × 105 EL4-huCD20 cells on day 0 and then received CAT-13 mAb therapy. Untreated mice (□, n = 6) were also used as controls (log-rank analysis, *P = .01). (B) Surviving CAT-13–treated mice received intraperitoneal injections of GK1.5 mAb starting on day 69 (
, n = 12) and nondepleted control mice (■, n = 12) were then injected with 5 × 105 EL4-huCD20 cells on day 0 and then received CAT-13 mAb therapy. Untreated mice (□, n = 6) were also used as controls (log-rank analysis, *P = .01). (B) Surviving CAT-13–treated mice received intraperitoneal injections of GK1.5 mAb starting on day 69 ( , n = 9) or not (■, n = 9) and were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 5) were used as controls (log-rank analysis, **P = .005). (C) Wild-type (n = 7) and CD8 KO C57Bl/6 mice (n = 18) were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells. Wild-type animals received the CAT-13 mAb therapy (□, n = 7). CD8 KO mice were divided into 2 groups. The first group was left untreated and used as a control (▷, n = 6). The second group of CD8 KO mice received the CAT-13 mAb therapy (◀, n = 18; log-rank analysis, †P = .01; ††P < .001). (D) Both wt (■, n = 4) and CD8 KO (◀, n = 10) surviving CAT-13–treated animals were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 6) were used as controls (log-rank analysis, †††P = .02). Black arrows indicate CAT-13 mAb injections.
, n = 9) or not (■, n = 9) and were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 5) were used as controls (log-rank analysis, **P = .005). (C) Wild-type (n = 7) and CD8 KO C57Bl/6 mice (n = 18) were intravenously injected on day 0 with 5 × 105 EL4-huCD20 cells. Wild-type animals received the CAT-13 mAb therapy (□, n = 7). CD8 KO mice were divided into 2 groups. The first group was left untreated and used as a control (▷, n = 6). The second group of CD8 KO mice received the CAT-13 mAb therapy (◀, n = 18; log-rank analysis, †P = .01; ††P < .001). (D) Both wt (■, n = 4) and CD8 KO (◀, n = 10) surviving CAT-13–treated animals were intravenously challenged with 5 × 105 EL4-huCD20 cells on day 70. Untreated mice (□, n = 6) were used as controls (log-rank analysis, †††P = .02). Black arrows indicate CAT-13 mAb injections.
Because CD8+ T cells are potent tumor cell killers, we also analyzed the capacity of CAT-13 mAb to induce long-lasting antitumor protection in C57Bl/6 mice without CD8+ cells. The identical overall survival in both CD8 KO and wt mice indicates that effective mAb therapy did not require CD8+ T cells at treatment onset (Figure 5C; P < .001). CD8 KO and wt treated mice that survived the initial tumor inoculation were then intravenously challenged on day 70 with 5 × 105 EL4-huCD20 cells. In contrast to the wt mice, all CD8 KO mice died after the tumor challenge (Figure 5D), at a rate similar to that of wt untreated control mice after the initial tumor injection. This finding shows that CD8+ cells are required for antitumor protection after tumor challenge.
Altogether, these results provide evidence that the CAT-13 mAb induction regimen triggers an antitumor cellular immune response that requires the presence of CD4+ cells at the beginning of mAb therapy and both CD4+ and CD8+ cells after tumor challenge.
The addition of IL-2 to anti-huCD20 mAb therapy improved overall survival after tumor challenge
Because IL-2 enhances T-cell antitumor activity in mouse and human cancers,28,29 we evaluated the effect of its association with CAT-13 mAb therapy on overall survival. To rule out the hypothesis of a direct effect by IL-2 on tumor cells, we incubated EL4-huCD20 cells in vitro with various concentrations of IL-2 and assessed cell viability and CD25 expression. In no test conditions did we see either enhanced EL4-huCD20 cell death or up-regulation of CD25 expression after incubation (data not shown).
IL-2 was first administered concomitantly with CAT-13 mAb (ie, 5 intraperitoneal injections of CAT-13 mAb from day 1 to day 13 and 2 intraperitoneal injections of 100 000 IU of IL-2 on days 1 and 4). Overall survival was similar in the mice treated with CAT-13 alone and with IL-2 and CAT-13 (Figure 6A). Second, we evaluated the effect of IL-2 when injected into surviving CAT-13–treated mice after the tumor challenge on day 70 (ie, 2 intraperitoneal injections of 100 000 IU of IL-2 on days 71 and 74; Figure 6B). In 3 independent experiments, the overall survival of the CAT-13–treated mice receiving IL-2 was higher than that of animals without IL-2 injections after the tumor challenge (Table 2; P = .004). Thus, IL-2 therapy improved the overall survival rate of tumor-challenged mice only when it was administered after the tumor challenge. These results support the existence of a T cell–dependent antitumor immune response that is triggered by the CAT-13 induction regimen and can be potentiated by IL-2.
Increase of the overall survival rate by IL-2 therapy given after tumor challenge in vivo. (A) Mice were intravenously injected on day 0 with EL4-huCD20 cells (n = 24) and divided into 3 groups. The first group (□, n = 8) was used as a control group and left untreated. Mice from the second group (■, n = 16) received the CAT-13 mAb therapy. Mice from the third group (gray pentagons, n = 8) received the CAT-13 mAb therapy associated with 2 intraperitoneal injections of IL-2 on days 1 and 4 (log-rank analysis, *P < .001; **P < .001). (B) Surviving CAT-13–treated mice (n = 10) were challenged intravenously with EL4-huCD20 cells on day 70 and divided into 2 groups. The first group was left untreated (■, n = 5). The second group was subsequently intraperitoneally injected with IL-2 on days 71 and 74 (gray pentagons, n = 5). Naive mice injected with EL4-huCD20 cells (□, n = 5) were used as controls (log-rank analysis, †P = .002; ††P = .002). Black arrows indicate CAT-13 mAb injections; and gray arrows, IL-2 injections.
Increase of the overall survival rate by IL-2 therapy given after tumor challenge in vivo. (A) Mice were intravenously injected on day 0 with EL4-huCD20 cells (n = 24) and divided into 3 groups. The first group (□, n = 8) was used as a control group and left untreated. Mice from the second group (■, n = 16) received the CAT-13 mAb therapy. Mice from the third group (gray pentagons, n = 8) received the CAT-13 mAb therapy associated with 2 intraperitoneal injections of IL-2 on days 1 and 4 (log-rank analysis, *P < .001; **P < .001). (B) Surviving CAT-13–treated mice (n = 10) were challenged intravenously with EL4-huCD20 cells on day 70 and divided into 2 groups. The first group was left untreated (■, n = 5). The second group was subsequently intraperitoneally injected with IL-2 on days 71 and 74 (gray pentagons, n = 5). Naive mice injected with EL4-huCD20 cells (□, n = 5) were used as controls (log-rank analysis, †P = .002; ††P = .002). Black arrows indicate CAT-13 mAb injections; and gray arrows, IL-2 injections.
Discussion
Despite the clinical efficacy of rituximab, a complete and durable response in patients treated with this mAb remains rare.4,30,31 Potential factors thought to influence the efficacy of rituximab in vivo include both host- and tumor-related resistance mechanisms.32,33 However, another important factor that might affect this is the capacity of some rituximab-treated patients to develop a long-lasting antitumor immune response.
We showed in this study that an induction regimen with anti-huCD20 mAb protects mice injected with EL4-huCD20 tumor cells from a subsequent tumor challenge (Figure 1). The lack of protection for the surviving mice when the tumor challenge uses EL4-wt cells indicates, however, that this protection is huCD20-specific. This CAT-13–induced antitumor protection is probably not the result of the induction of an endogenous antibody response. The only anti-EL4–huCD20 immunoglobulin detected in the sera of CAT-13–treated mice was IgG2a (Figure 3A; and data not shown). Their binding to EL4-huCD20 decreased over time, even after the tumor challenge on day 70, thereby suggesting that these IgG2a correspond to small amounts of CAT-13 mAb in the blood of mAb-treated mice.
Several findings make it unlikely that this small amount of anti-EL4–huCD20 IgG2a accounts for the protection of animals after tumor challenge. First, recipient mice receiving pooled sera from surviving CAT-13–treated mice before the tumor cell injection all died, as did the untreated control mice (Figure 3B). Second, CAT-13 therapy with nonoptimal mAb doses (ie, 5 × 50 and 5 × 10 instead of 5 × 200 μg/mouse) led only to either a low overall survival rate (12.5% for the 5 × 50 μg dose) or to no protection at all (for the 5 × 10 μg dose; data not shown). Furthermore, the anti-EL4–huCD20-specific IgG2a serum levels on day 15 in these animals were higher or equal to those of mice treated with the optimal 5 × 200 μg dose on day 70 (data not shown). Taken together, these results suggest that the long-lasting antitumor protection is the result of neither the development of an endogenous antibody response against EL4-huCD20 nor the presence of small amounts of CAT-13 mAb in the blood of tumor-challenged-mice. Of note, it was previously reported that a protective antibody response against Friend leukemia cells is elicited in mice after treatment with mAbs directed against Friend leukemia virus envelope gp70 antigen.24 These opposite results might reflect differences in the nature of the target antigen, tumor type, or the presence of Friend leukemia virus.
In vitro cytotoxicity experiments and in vivo adoptive transfer experiments (Figure 4A-B), both performed with spleen cells from surviving CAT-13–treated mice, suggest that a cellular immune response occurred after mAb therapy. Depletion experiments demonstrated that a long-term protective effect requires CD4+ cells both at the beginning of the treatment (initial phase) and at tumor challenge (late phase; Figure 5A-B). The lower absolute number of these CD4+ cells among CD3+ spleen cells in untreated mice, compared with their normal level after mAb therapy (Figure 4C), indicates that this therapy affects the splenic CD4+ T-cell subset. In contrast, we showed with CD8 KO mice that CD8+ cells were required only at the late phase of antitumor protection (Figure 5C-D). Thus, although CD4+ cells are needed during both initial and late phases, CD8+ cells are required only after tumor challenge to achieve long-lasting protection.
All these data led us to hypothesize a sequential mechanism for the long-lasting mAb-induced antitumor protection. Anti-huCD20 mAb, through its Fc portion, may recruit and activate FcγR-bearing cytotoxic innate effectors and antigen-presenting cells during the initial phase of the protection. This might then elicit a potent activation of CD4+ T cells, as previously discussed.34 These T cells might then act as direct antitumor killer effectors35 or as classic T helper cells that enhance the antitumor activity of innate effectors, or both. Recent reports that CD4+ cells can be more effective than CD8+ cells in controlling tumor development, perhaps through the activation of innate effectors, support this hypothesis.36,37 The innate phagocyte network, rather than CD8+ T cells, might play an essential role in EL4-huCD20 cell depletion, as previously reported in animal models of mAb-mediated B-cell depletion.38,39 In contrast, during the late phase, when the mAb concentration is very low, poorly opsonized tumor cells might not be captured by phagocytes. Instead, antitumor memory CD4+ T cells generated during the initial phase might activate CD8+ tumor-specific cytotoxic T lymphocytes, leading to tumor control or eradication.
In our study, no antigen spread occurred after the anti-huCD20 mAb therapy, as shown when EL4-wt cells were used for the tumor challenge (Figure 1B). A recent report showed that the combined use of GM-CSF and mAb has the potential to overcome tolerance to self-antigens.40 Early administration of GM-CSF together with anti-CD20 mAb could therefore potentiate antigen-presenting cell activity and enhance T-cell priming, ultimately leading to the breakdown of tolerance. This hypothesis is consistent with the recent observation that the combined use of rituximab and GM-CSF produces high response rates in patients with relapsed or progressive FL.18 Another clinical study suggests that rituximab with interferon-α2a improves both the quality and duration of the responses in relapsing FL patients.17 Another approach to strengthening the efficacy of anti-CD20 therapy might be to combine anti-CD20 with cytokines acting on tumor-specific effector cells at a later stage of the therapeutic scheme, once these cells have been recruited and activated after the induction regimen. We demonstrated here that IL-2 treatment increased the overall survival of CAT-13–treated mice when infused after tumor challenge, but not when injected in association with CAT-13 mAb during the induction regimen (Figure 6A-B; Table 2). These data show that IL-2, used after the establishment of the antitumor protection, enhances the overall survival rate. This effect could be the result of a boost of tumor-specific effector T cells induced during the initial phase of mAb therapy. This phenomenon appears somewhat similar to the adjuvant effect of IL-2 in antitumor vaccination.28,29,41,42 It should be pointed out that IL-2 injection did not lead to a decrease in the overall survival rate at any time of administration. This suggests, in contrast to some other studies,43,44 that no regulatory T cell–mediated suppression was induced.
In conclusion, anti-CD20 mAb therapy induces a cellular immune response that leads to long-lasting in vivo antitumor protection. As recently discussed by Weiner et al,45 the promotion of an endogenous antitumor immune response in mAb-treated cancer patients is becoming a major goal for improving mAb-based therapies. This challenge could be addressed in the near future either by rational molecular engineering of mAbs or by their combined use with immunomodulatory molecules.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ion Gresser for helpful discussion.
This work was supported by Inserm and Laboratoire français du Fractionnement et des Biotechnologies. R.A. was supported by Laboratoire français du Fractionnement et des Biotechnologies/ANRT (CIFRE fellowship no. 173/2006).
Authorship
Contribution: R.A. and J.-L.T. designed research, analyzed data, and wrote the paper; R.A. and E.G. performed research and collected and analyzed data; and W.H.F. revised the paper.
Conflict-of-interest disclosure: W.H.F. is the President of the Laboratoire français du Fractionnement et des Biotechnologies Scientific Advisory Board. The remaining authors declare no competing financial interests.
Correspondence: Jean-Luc Teillaud, Team 14 Antibody BioEngineering, Inserm U.872/Cordeliers Research Center, 15, rue de l'Ecole de Medecine, F-75006 Paris, France; e-mail: jean-luc.teillaud@crc.jussieu.fr.