In this issue of Blood, Yong and colleagues report that circulating blood platelets and monocytes in stable coronary artery disease patients can become activated by shear stress during passage through a stenosed sclerotic lesion, despite dual antiplatelet therapy, and the absence of either plaque rupture or collagen exposure.1
A remarkable feature of the Yong et al study was the collection of blood samples from the coronary artery both upstream and downstream of the coronary lesion, as well as from the coronary sinus. These multiple samples were combined with the application of new computational angiographic imaging technology so that cellular/molecular changes in platelets or monocytes could be correlated with the severity of stenosis and the calculated degree of intravascular shear stress. Blood platelets from 20 patients with stable angina undergoing elective percutaneous coronary interventions involving an epicardial coronary artery showed significantly elevated surface expression of the α-granular activation marker, P-selectin, and increased platelet-monocyte aggregates downstream of the stenosis.1 There was no significant increase in the platelet levels of activated integrin αIIbβ3 which binds von Willebrand factor (VWF) or fibrinogen in platelet aggregation, possibly because all the patients studied were on the antiplatelet drugs, aspirin and clopidogrel (Plavix). The data suggest that levels of shear stress under these conditions are sufficient to activate platelets leading to degranulation and monocyte interaction, but without shear-induced platelet aggregation, raising important questions regarding the efficacy of antiplatelet drugs in cardiovascular disease.
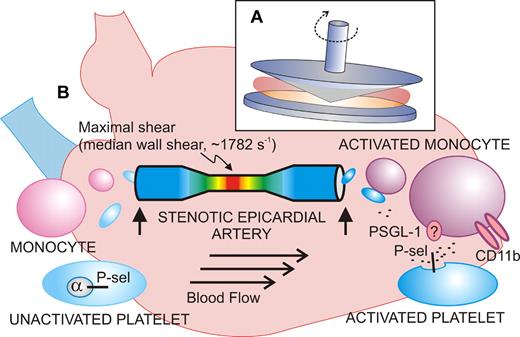
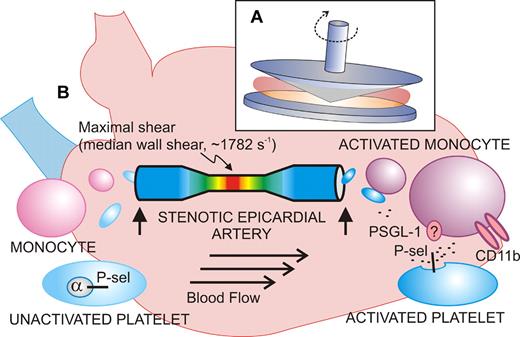
Shear-induced activation of vascular cells in vitro and in vivo. (A) Diagram of the cone-plate viscometer, where a rotating cone on a fixed plate exerts uniform shear stress on a sample of blood or platelets without exposure to a thrombogenic surface. (B) Shear stress–exposed blood passed through a stenotic coronary artery shows signs of activated platelets (increased P-selectin expression) and activated monocytes (increased CD11b) when blood samples (vertical arrows) are taken upstream or downstream of the atherosclerotic lesion from stable angina patients. The extent of stenosis and shear rates are calculated using computational angiographic imaging.
Shear-induced activation of vascular cells in vitro and in vivo. (A) Diagram of the cone-plate viscometer, where a rotating cone on a fixed plate exerts uniform shear stress on a sample of blood or platelets without exposure to a thrombogenic surface. (B) Shear stress–exposed blood passed through a stenotic coronary artery shows signs of activated platelets (increased P-selectin expression) and activated monocytes (increased CD11b) when blood samples (vertical arrows) are taken upstream or downstream of the atherosclerotic lesion from stable angina patients. The extent of stenosis and shear rates are calculated using computational angiographic imaging.
Activation of blood platelets by rheologic shear stress has been known for decades, stemming from studies of artificial heart valves, and leading to the use of experimental devices like the cone-plate viscometer (see figure).2 This device, involving a rotating cone on a flat plate, applies uniform shear stress to a sample at shear rates ranging from low to high physiologic (∼ 20-1600 s−1) or pathologic levels (> 10 000 s−1 in a stenotic artery). High shear induces conformational activation of the platelet receptor, glycoprotein Ibα (GPIbα; the VWF-binding subunit of GPIb-IX-V), and VWF, leading to platelet activation, secretion of adenosine diphosphate (ADP), thromboxane A2 (TXA2) production, and activation of αIIbβ3. In this experimental setup, shear-induced platelet aggregation occurs without exposure to a thrombogenic surface, and GPIbα, VWF, ADP, and αIIbβ3 are indispensable.2 Recent studies reveal how shear stress can alter conformation of the GPIbα ligand-binding domain to enhance GPIbα-VWF bond strength,3 helping to explain how platelet adhesion and activation can occur at pathologic shear rates in flowing blood. Yong and colleagues also exposed blood from healthy individuals (collected from an arm vein) to shear rates of 1800 s−1 for 5 minutes in a cone-plate viscometer and showed increased platelet P-selectin expression and platelet-monocyte interactions comparable with in vivo results (median wall shear rate, 1782 s−1). However, while an inhibitory anti–P-selectin antibody completely blocked platelet-monocyte interaction, it did not affect the shear-induced increase in monocyte CD11b expression. CD11b (the α-subunit of the leukocyte integrin, αMβ2; CD11b/CD18; Mac-1) is up-regulated upon cellular activation and mediates adhesion to endothelial cells via intercellular adhesion molecule-1 (ICAM-1) or platelets via GPIbα.4,5 This finding suggests not only that platelet P-selectin mediates the interaction between platelets and monocytes, possibly involving its counterreceptor P-selectin glycoprotein ligand-1 (PSGL-1),5 but, in addition, that monocytes also respond to shear independent of platelet adhesion. It is known that shearing leukocytes alters PSGL-1 surface distribution to microvilli, facilitating cell rolling and attachment, as well as up-regulating CD18 expression.6
Recent studies suggest shear stress alone can provoke aggregation of discoid platelets unrelated to aggregation involving ADP/TXA2 secretion.7 The present study uniquely demonstrates platelet activation in vivo due to vascular stenosis, correlating with the extent of stenosis and levels of shear, but without a requirement for secondary platelet agonists (patients were treated with aspirin that blocks TXA2 production and clopidogrel that blocks the platelet ADP receptor, P2Y12). Despite no significant activation of αIIbβ3, this level of platelet activation could increase propensity for thrombosis and thrombotic risk, or else promulgate the inflammatory component of cardiovascular disease through activation of leukocytes (involving P-selectin or other platelet-derived proinflammatory factors).5 Increased P-selectin expression also implies release of other α-granule constituents, including PF4, adhesive proteins, mitogenic and angiogenic factors, coagulation factors, and fibrinolytic inhibitors.5 P-selectin is known to increase generation of tissue-factor-bearing, procoagulant microparticles from monocytes which can also increase thrombotic risk.8
Notwithstanding the potential technical and clinical challenges discussed by Yong et al, the findings raise many interesting mechanistic questions, and have significant clinical and therapeutic implications. Blood cells presumably circulate through the coronary vascular bed multiple times. How, then, can changes in activation be detectable in samples collected proximal and distal to the lesion (that is, after a single passage)? Are the shear-dependent effects on platelets and monocytes cumulative, reversible, or both, or is there a transient cellular “memory” of brief exposure to elevated shear? In this regard, it is known that platelets exposed to transient shear stress in the cone-plate viscometer retain the capacity to support VWF binding to GPIbα even after cessation of shear.9 Another possibility is that shear-exposed platelets are more susceptible to clearance from the circulation, through interactions with leukocytes or other cells, or through GPIbα-dependent interaction with αMβ2 on phagocytic cells, a mechanism involved in clearance of chilled platelets in the liver.10
Finally, a significant aspect of this study is what it reveals about conventional antiplatelet therapy (aspirin, clopridogrel). These drugs inhibit activation of αIIbβ3, and inhibition of αIIbβ3-dependent platelet aggregation under static or shear conditions represents the usual criteria for selection of antiplatelet drugs (including αIIbβ3 inhibitors). Although blockade of TXA2, and ADP receptors P2Y1 and P2Y12, inhibited shear-induced platelet P-selectin expression and platelet-monocyte aggregation in healthy donor samples in vitro, therapeutic doses of aspirin/clopidogrel did not block measurable shear-induced platelet activation (indicated by P-selectin expression) on passage through a stenotic artery in cardiovascular patients in vivo.1 Therefore, while antiplatelet drugs may reduce thrombotic risk, the present study emphasizes the need for a wider selection of drugs to attenuate pathologic platelet function. This novel analysis of shear-dependent effects on vascular cells in vivo also highlights the need for better bioassays of platelet function that take pathologic shear stress into account, and could lead to the identification of safer therapeutic targets and inhibitors.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■