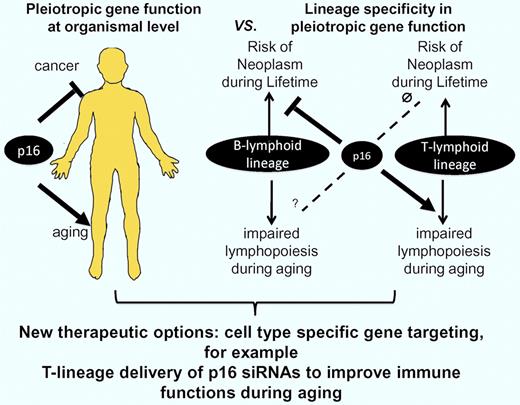
In this issue of Blood, Liu et al provide the first genetic evidence that p16 defines a lymphoid lineage intrinsic gatekeeper, which prevents B-lymphocyte transformation but impairs T-lymphocyte function in aging mice.1
Imagine if we could influence our immune system to produce youthful numbers of highly functional lymphocytes throughout life up to a very advanced age, without increasing the risk of tumors derived from the lymphoid lineage. It is conceivable that such an intervention would reduce the risk of deleterious infections and cancer.2 To therapeutically target the immune system to prevent aging-associated declines in immune function, it is of utmost importance to identify molecular causes of this process.
Molecular mechanisms that limit cell proliferation (eg, telomere shortening) have been implemented in cancer protection, but were also shown to contribute to the decline in tissue maintenance and regeneration during aging.3 The cycling-dependent kinase inhibitor p16 represents one example of a cell-cycle inhibitor, which has not only been implemented to prevent cancer formation at early age, but also to impair stem cell function and tissue maintenance during aging.4 The concept that biologic processes, regulating organismal function (such as the expression of specific genes), can have pleiotropic effects during lifetime (tissue protective during early life but tissue destructive in late life) has led to the theory of “antagonistic pleiotropy” in aging.5,6 It remains an open debate whether genes that exhibit a pleiotropic function during lifetime could represent targets for novel therapies, aiming to prevent the evolution or progression of age-associated organ dysfunction. In this regard, it is an important question whether lifecycle-dependent pleiotropic effects of genes occur within a single cell lineage or in separate lineages. If the latter holds true, a pleiotropic tumor suppressor gene may prevent tumor formation in one cell lineage and induce the evolution of age-associated dysfunction in a different lineage. In the case of lineage-specific pleiotropy, cell type–specific gene targeting could improve age-associated impairments in maintenance of one tissue without affecting gene expression and cancer risk in other tissues.
To investigate the role of p16 in lymphocyte aging and transformation, Liu et al analyzed aging mouse cohorts carrying B or T lymphocyte–specific deletions of the INK4A gene locus encoding for p16 protein compared with a wild-type cohort. The authors demonstrate that p16 deletion in T-lymphocyte progenitor cells impairs age-dependent involution of the thymus and improves the production of naive and memory T lymphocytes in aging mice. Importantly, this enhancement in age-dependent T-cell production was associated with improved immune responses in aging mice and did not increase the cancer risk. These results provide a first example of a T cell–specific gene knockout impairing age-dependent declines in thymopoiesis. The findings do not argue against inhibitory effects of the aging environment (thymic niche or systemic blood circulation) on T lymphogenesis. It is conceivable that inhibitory effects of the aging environment on T lymphogenesis could lead to a cell-intrinsic up-regulation of p16 in T-lymphocytic progenitor cells. An important question in future studies is to delineate the molecular pathways that control the up-regulation of p16 in T-lymphocyte progenitor cells during aging.
In sharp contrast to the beneficial effects of p16 deletion in the T-lymphocyte lineage, Liu et al demonstrate that the deletion of p16 in B-lymphocyte progenitor cells led to an early formation of B cell–derived neo plasms in middle-aged mice. Positive effects of p16 deletion on the prevention of B-lymphocyte aging could not be observed in this time window. The contrasting effects of p16 deletion on aging and transformation in the B-lymphopoietic versus T-lymphopoietic cells represent the most important finding of the current study. To our knowledge, these data provide the first experimental evidence that lineage-specific deletion of a single gene (p16) can have age-dependent, pleiotropic effects, improving maintenance and function in one compartment (T lmphocytes), but promoting tumorigenesis in another compartment (B lymphocytes). The authors conclude from their findings that “this work serves as a cautionary tale for those who would seek to ameliorate aging by globally attenuating tumor suppressor function.”1 This conclusion is important, but to see it more positively we can also conclude from these data that cell type–specific targeting of pleiotropic tumor suppressor genes could represent a novel therapeutic concept for the improvement of tissue maintenance and function during aging (see figure). In addition, it should be noted that the deletion of tumor suppressor genes (p21 or Exonuclease-1) at the level of the whole organism (germline deletion) has been shown to elongate the lifespan of prematurely aging mice with dysfunctional telomeres.7,8 These data indicate that a systemic inactivation of tumor suppressor genes could have beneficial effects at advanced age, when defects in organ maintenance become life limiting.
It has been shown that tumor suppressor genes (eg, p16) have pleiotropic effects during lifetime preventing the formation of cancer during early life but contributing to impairments in stem-cell function and organ maintenance during aging. Liu et al provide the first experimental evidence for lineage-specific pleiotropic effects of p16 in the aging immune system. B lineage–specific deletion of p16 leads to B cell–derived neoplasms in middle-aged mice without improving B lymphopoiesis at this age. In contrast, T lineage–specific deletion of p16 does not lead to tumorigenesis but significantly improves T lymphopoiesis and immune functions in aging mice. The data indicate that p16 does not contribute to tumor suppression in T-lymphoid cells but has an important tumor-suppressive role in B-lymphoid cells. In contrast, p16 contributes to impairments in T lymphopoiesis during aging but has no significant effects on B lymphopoiesis in middle-aged mice. Inhibitory effects of p16 on B lymphopoiesis at advanced age cannot be excluded. The data support a new concept indicating that lineage-specific gene-targeting (targeting of p16 in T-lymphoid lineage) could represent a therapeutic option to improve tissue maintenance during aging (T lymphopoiesis) without affecting gene expression and transformation in other compartments (B-lymphoid lineage).
It has been shown that tumor suppressor genes (eg, p16) have pleiotropic effects during lifetime preventing the formation of cancer during early life but contributing to impairments in stem-cell function and organ maintenance during aging. Liu et al provide the first experimental evidence for lineage-specific pleiotropic effects of p16 in the aging immune system. B lineage–specific deletion of p16 leads to B cell–derived neoplasms in middle-aged mice without improving B lymphopoiesis at this age. In contrast, T lineage–specific deletion of p16 does not lead to tumorigenesis but significantly improves T lymphopoiesis and immune functions in aging mice. The data indicate that p16 does not contribute to tumor suppression in T-lymphoid cells but has an important tumor-suppressive role in B-lymphoid cells. In contrast, p16 contributes to impairments in T lymphopoiesis during aging but has no significant effects on B lymphopoiesis in middle-aged mice. Inhibitory effects of p16 on B lymphopoiesis at advanced age cannot be excluded. The data support a new concept indicating that lineage-specific gene-targeting (targeting of p16 in T-lymphoid lineage) could represent a therapeutic option to improve tissue maintenance during aging (T lymphopoiesis) without affecting gene expression and transformation in other compartments (B-lymphoid lineage).
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■