In this issue of Blood, Nelson and colleagues1 and Warsch and colleagues2 report that signal transducers and activators of transcription 5 (STAT5) is an attractive target to circumvent tyrosine kinase inhibitor (TKI) resistance in chronic myeloid leukemia (CML).
BCR-ABL activate many signaling pathways in leukemic cells, such as RAS, PI-3K and NF-κB. STAT5 was one of the first pathways to be described as being constitutively activated by p210 BCR-ABL and p190 BCR-ABL.3-5 STAT5 activation has been shown to be correlated with functional effects such as antiapoptosis through activation of Bcl-XL6 and drug resistance phenotype through activation of Rad51.7 BCR-ABL directly induces a tyrosine-phosphorylation and dimerization of STAT5 followed by nuclear translocation of the STAT5 dimers that then bind to consensus sequences through their DNA binding domain and promote activation of downstream target genes (see figure). In several experimental systems, STAT5 activation has been shown to be absolutely essential for leukemic cell survival,8 but there is no clear data with regard to its involvement in tyrosine kinase inhibitor-resistance of CML cells. The studies by Nelson et al1 and Warsch et al2 explore this important issue.
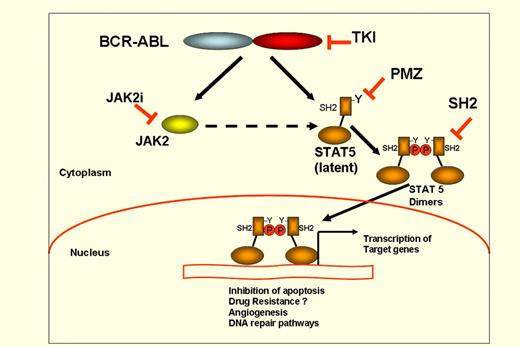
Schematic representation of STAT5 activation in CML ands strategies of STAT5 inhibition. BCR-ABL phosphorylates STAT5 at a critical Tyrosine (Y) residue close to SH2 domain, with subsequent dimerization and nuclear translocation of STAT5:STAT5 dimers, which bind to DNA at consensus sequences of the target gene promoters. PMZ indicates Pimozide; TKI, tyrosine kinase inhibitors; SH2i, STAT5 inhibitors targeting SH2 domain; and JAK2i, Jak2 inhibitors.
Schematic representation of STAT5 activation in CML ands strategies of STAT5 inhibition. BCR-ABL phosphorylates STAT5 at a critical Tyrosine (Y) residue close to SH2 domain, with subsequent dimerization and nuclear translocation of STAT5:STAT5 dimers, which bind to DNA at consensus sequences of the target gene promoters. PMZ indicates Pimozide; TKI, tyrosine kinase inhibitors; SH2i, STAT5 inhibitors targeting SH2 domain; and JAK2i, Jak2 inhibitors.
In the work by Nelson and colleagues, the authors used cell lines stably transfected with STAT5-responsive elements controlling a reporter (luciferase) gene and screened a chemical library. They identified the neuroleptic drug pimozide as a compound that appeared to specifically inhibit STAT5 phosphorylation (see figure) with no effect on STAT1 or NF-κB. This drug is a compound that has current Food and Drug Administration approval for the treatment of tics in Tourette syndrome.9 They showed that it acts in synergy with imatinib and nilotinib and surprisingly does not induce any dephosphorylation of BCR-ABL. On pimozide treatment there is also a down-regulation of the expression of several STAT5 target genes demonstrating a significant downstream effect. It appears therefore that pimozide is not a TK inhibitor but rather acts mainly by dephosphorylating STAT5. To determine the potential use of this compound to target specifically leukemic cells, experiments undertaken in cell lines have shown that pimozide has cytotoxic activity in leukemic cell lines but no cytotoxic effect in normal cells. Interestingly, pimozide also has growth inhibitory activity in CML cells with T315I mutation that confers resistance to all 3 TKIs currently in clinical use. Finally, pimozide also has activity against clonogenic CD34+ CML cells while sparing normal clonogenic CD34+ cells. The growth inhibitory effect of pimozide in CML cells seems to be the result of both cell-cycle arrest and increased apoptosis. The mechanism of action of the drug on STAT5 remains unclear; it remains to be determined if it involves the activation of a phosphatase that dephosphorylates STAT5, leading to its inactivation.
The role of STAT5 in TKI resistance in CML has also been studied by Warsch and colleagues, using a different approach. These authors used v-ABL transformed cell lines in which they overexpressed STAT5, STAT1, or STAT3. They show that STAT5 overexpression leads clearly to a TKI-resistant phenotype whereas STAT3 and STAT1 have no effect. High levels of STAT5 protected leukemic cells from TKI toxicity in the absence of JAK2 expression, suggesting that BCR-ABL (or v-ABL) induced JAK2 phosphorylation is not required for STAT5 activation in leukemic cells. The correlation with STAT5 expression and TKI sensitivity was further demonstrated using bone marrow cells from STAT5null/+ mice that express lower levels of STAT5 compared with wild-type STAT5+/+ mice. After retrovirus-mediated BCR-ABL gene transfer, STAT5null/+ cells exhibited increased sensitivity to TKI compared with their counterparts expressing higher levels of STAT5. Similarly, overexpression of STAT5A in v-ABL transformed B cells followed by imatinib treatment in vitro or in vivo led to imatinib-resistance only in cells with high STAT5A expression. Finally, in primary CML samples from patients in advanced CML, high levels of STAT5 mRNA levels have also been shown to correlate with TKI resistance and accordingly, STAT5A and STATB expression (2 highly homologous STAT5 gene products) were found to be increased in CML patients with advanced stage, with or without ABL-kinase mutations. Overall, these results, in agreement with the report of Nelson et al,1 suggest that STAT5 phosphorylation is a marker of CML progression and it could be an attractive target to circumvent TKI resistance in CML.
The introduction of imatinib mesylate as a first line of therapy for CML has profoundly changed the prognosis of the disease but resistance occurs in 15%-20% of cases,10 and most importantly, the most primitive CML stem cells exhibit several mechanisms of resistance to TKI therapies.11 This explains the recent considerable interest in finding novel targets in TKI-resistant CML cells using secondary, BCR-ABL–dependent or –independent signaling pathways. Such an approach is interesting if a clinically useful drug can be identified and that can be given without major adverse events to CML patients on TKI therapy. What are the possibilities that STAT5 could be a good target in CML? The persistence of low levels of phosphorylated STAT5 leading to increased viability of leukemic cells is an interesting concept especially in the context of bone marrow niche, which provides the leukemic cells with survival and/or quiescence signals. However, one of the important questions with STAT5 targeting will be the evaluation of a specific therapeutic window allowing targeting of leukemic cells without harming normal cells. In this context, the work by Nelson and colleagues clearly shows a selective effect with regard to leukemic progenitors compared with normal progenitors. From the clinical point of view, it will be important to determine the potential additive side effects of a given TKI and a drug such as pimozide, which might have cardiovascular and neurologic side effects. A clinical trial combining a TKI and pimozide could investigate both the toxicity and the efficacy of the combined therapy using molecular monitoring.
One crucial question that has not been addressed is the effects of pimozide in primitive BCR-ABL–expressing hematopoietic stem cells. Similarly, it is not known if STAT5A/B expression in primitive hematopoietic stem cells correlates with resistance of these cells to TKI and if the selective effects observed in v-ABL–transformed cells can be translated to human CML. The recent demonstration that survival and growth of the most primitive CML stem cells are not dependent on BCR-ABL TK activity12 suggests that targeting CML stem cells by other pathways (such as Sonic Hedgehog that has been shown to be required for maintenance of CML stem cells) will be needed to obtain complete eradication of malignant cells. A drug such as pimozide, which seems to inhibit the growth of the CML cells without affecting TKI activity of BCR-ABL, could be of major interest if it has significant activity at the level of CML stem cells. Other strategies of STAT5 inhibition in CML could involve the use of STAT5-inhibitors targeting SH2-domain or JAK2 inhibitors, although there is clear evidence that STAT5 phosphorylation by BCR-ABL can be a JAK2-independent event. Overall, the 2 works reported here add new investigative avenues to the very active research field in the targeted therapies of CML13 and should contribute to the final task of curing CML.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal