Abstract
The integrin lymphocyte function-associated antigen 1 (LFA-1) controls many functions of T lymphocytes and is particularly essential during lymphocyte migration from blood into tissues. LFA-1 is considered to initiate “outside-in” signaling when bound to ligand intercellular adhesion molecule 1 (ICAM-1), but little is known about the proteins involved or where in the cell such LFA-1–mediated signaling might be operating. Here we show that LFA-1 is constitutively associated with the protein tyrosine kinases Lck and zeta chain–associated protein of 70 kDa (ZAP-70). When LFA-1 binds ICAM-1, both kinases become phosphorylated and the consequence of kinase activation is the conversion of intermediate- to high-affinity LFA-1 and an increase in close contact with ICAM-1. In the polarized T lymphocyte, phospho-ZAP-70 is concentrated within a region of high-affinity LFA-1 that includes talin and encompasses the lamella/lamellipodial interface as well as further back in the cell. Deficiency of ZAP-70 through inhibition or knockdown in T lymphocytes decreases the speed of migration on ICAM-1, as well as reducing firm adhesion under shear-flow conditions. Through its control of high-affinity LFA-1, the LFA-1/Lck/ZAP-70 complex is in position to initiate the rapid adhesion strengthening and migration necessary for T-lymphocyte responses when stimulated vasculature is encountered at sites of infection or injury.
Introduction
A successful immune response is critically dependent on the ability of leukocytes to leave the circulation by migrating across the vasculature into a lymph node or infected tissue site.1-3 The blood-to-tissue transition occurs through a well-defined sequence of adhesive events along the vessel wall involving selectins and integrins during which the leukocyte gradually slows down, arrests, and transmigrates into a tissue space. In this context, the rapid rolling phase is mediated by selectin engagement and the arrest and migration phase by integrins. The binding of E-selectin to its ligands on the neutrophil provides signals to activate integrin, converting fast to slow rolling4,5 or, alternatively, signaling through receptors such as chemokine or T-cell receptors can induce rapid integrin-mediated attachment of T cells.6 Whether the core signaling components of these individual “inside-out” pathways are identical for each mode of stimulation leading to active integrin is not yet certain.7
For a leukocyte such as a T cell, the β2 integrin lymphocyte function-associated antigen-1 (LFA-1) is the major integrin participating in these events.3,8 Inside-out signaling causes the bent, inactive form of LFA-1 to extend, making its intercellular adhesion molecule 1 (ICAM-1)–binding I domain accessible.9 This conformation represents intermediate-affinity LFA-1. Further change to the I domain region generates a third form of LFA-1 with higher affinity for ligand. Our previous work shows that migrating T cells express both intermediate- and high-affinity LFA-1, with each form localized to a distinct region of the T cell. Intermediate-affinity LFA-1 is associated with the lamellipodium at the dynamic leading edge, whereas high-affinity LFA-1 is clustered in a highly adhesive mid-cell focal zone corresponding to the lamellar region.10,11
When exposed to shear force resembling that experienced by leukocytes in the circulation, T cells use LFA-1 to attach with subsecond timing to ICAM-1.6,12 Integrins are allosteric receptors and it is considered that exposure to shear force contributes to formation of a high-affinity conformation.13,14 Such a switch is, however, insufficient on its own to generate cell spreading and motility.15 For these activities, signaling that leads to turnover in the cell's actin cytoskeleton and motor machinery is essential and, for T cells, this is a feature of LFA-1–mediated signaling.16-18
Integrins can signal into the leukocytes on which they are expressed but little is known about the “outside-in” signaling pathways associated with LFA-1 on T cells.3,19 For platelets and myeloid cells, Src family kinases are constitutively associated with integrins.20-22 Src binds directly to the β3 subunit of platelet integrin αIIbβ323 and Src family members Hck, Fgr and Lyn play similar but partially redundant roles in myeloid cells.24 The Src family member autophosphorylates after integrin binding to ligand.22,25 This can lead to phosphorylation of a nearby molecule containing an immunoreceptor tyrosine activation motif (ITAM) that serves as a docking site for the Src homology 2 (SH2) domains of Syk.26,27 For αIIbβ3 there is evidence that Syk can bind directly to the β3 subunit28,29 but may also require ITAM interaction for full activity.26 Src then phosphorylates Syk, a step that initiates a phosphorylation cascade of downstream effectors. This integrin-mediated signaling pathway closely resembles signaling through other immunoreceptors, in particular the antigen specific T-cell receptor (TCR) where Src kinases, Lck and Fyn, and Syk family kinase zeta chain–associated protein of 70 kDa (ZAP-70) have major roles.30-32
In this study, we have investigated the early stages of LFA-1 outside-in signaling in human T lymphocytes. We find that the tyrosine kinases Lck and ZAP-70 are constitutively complexed with LFA-1 and become phosphorylated and active after binding to ICAM-1. A focus on ZAP-70 reveals that it controls conversion of the intermediate- to high-affinity conformation of LFA-1 and is essential for the firm adhesion and migration of T cells under both nonshear and shear flow conditions.
Methods
Antibodies and reagents
Monoclonal antibodies (mAbs) used in this study are: YTH81.5 (CD11a nonfunction blocker),10 KIM127 (CD18 integrin extension reporter),33 24 (CD18 integrin activation reporter),34,35 all prepared at Cancer Research UK London Research Institute (CRUK LRI). The following mAbs/Abs were purchased: mAbs 8d4 (talin) and DM1A (α-tubulin; Sigma-Aldrich); ZAP-70 (BD Transduction Laboratories); Lck and Fyn (Santa Cruz Biotechnology); Abs phospho-ZAP-70 (Y319) and phospho-Src (Y516) that also detect phospho-Lck (Y394; Cell Signaling Technology). Alexa Fluor 488–phalloidin, Alexa Fluor 488– and Alexa Fluor 546–goat anti–mouse immunoglobulin G (IgG) were obtained from Invitrogen; Alexa Fluor 488 and Alexa Fluor 546 fluorochrome kits were from Invitrogen.
A pharmacologic inhibitor for the Src family, PP2, and negative control, PP3, were used at 40μM; the Syk family, piceatannol, at 10μM after titration (Calbiochem). Five domain ICAM-1–Fc was produced as previously described.10
Cell transfections
HSB2 T cells (2 × 107 cells) were washed in OptiMEM + GlutaMAX (Invitrogen) and electroporated with the following reagents using a Gene Pulser with Capacitance Extender (Bio-Rad UK) set at 960 μF and 260 mV: ZAP-70 small interfering (si)RNA (Dharmacon SMARTpool, M-005398-04) or control (Dharmacon SMARTpool, D-001810-10-05; Thermo Scientific); talin-1 siRNA (Ambion ID 5552 targeting exon 6) or control (Ambion ID 4611G) all at 400nM per reaction. Efficiency of individual siRNA knockdowns in T cells was evaluated by Western blotting. Alternatively, HSB2 T cells were transfected with full-length human ZAP-70–green fluorescent protein (GFP) or truncated (1-276) ZAP-70(SH2)2-GFP constructs (10 μg of cDNA per reaction). The GFP-positive cells were sorted before use with a MoFlo Cell Sorter (Beckman Coulter). Full details of the constructs, which were cloned in fusion with GFP into pCDNA3.1, will be reported elsewhere. Transfected cells were maintained in RPMI 1640 with 10% fetal calf serum (FCS) for up to 24 hours for ZAP-70 siRNA and cDNA transfected HSB2 cells and 48 hours for talin siRNA-transfected HSB2 cells.
ICAM-1–coated microspheres
Polystyrene microspheres (6μm; Polysciences Inc) were incubated with ICAM-1–Fc (200 μL, 8 μg of substrate) for 90 minutes at room temperature (RT). The beads were washed 3 times, in phosphate-buffered saline (PBS) and resuspended in 1 mL of 10 mg/mL bovine serum albumin (BSA) in PBS for 1 hour at RT and washed again. A total of 5 × 107 T blasts and 5 × 107 ICAM-1-Fc–coated or BSA-coated beads were washed into 500 μL of Hanks balanced salt solution (HBSS) with 5mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] for 30 minutes at 37°C. The cell-bead mixture was spun and resuspended in 1 mL of sample buffer. Lysates were filtered through a Millex GP Filter unit (0.22 μm) to remove bead debris and run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; NuPAGE, 4%-12% Bis-Tris gels; Invitrogen).
Interference reflection microscopy
T cells were plated on μ-slide IV (Ibidi) coated with 3 μg/mL ICAM-1-Fc as previously.10,11 Images of the close substrate contact of migrating cells were obtained at 20 minutes after initial attachment using a Zeiss Axiovert 100M inverted confocal microscope with a 63× NA/1.4 Plan-Apochromat oil-immersion objective lens. For evaluation of adhesion status, the area of contact within 20 nm of substrate level was measured using Metamorph Offline 7.1 software (n = 10 cells per sample type).
Flow assay
μ-Slide IV (Ibidi) was coated with 3 μg/mL ICAM-1–Fc as previously,10,11 then 10 μg/mL E-selectin–Fc for 1 hour at 37°C (R&D Systems Europe Ltd) followed by blocking 1 hour with 2% BSA. A shear force of 1 dyne/cm2 was imposed using an automated syringe pump (KDS model 200; Linton Instrumentation) and the activity of interacting T lymphoblasts was recorded using a Nikon Diaphot 300 microscope with a Sony XCD-X700 camera using a 20× lens and AQM2001 Kinetic Acquisition Manager software (Kinetic Imaging Ltd). Videos from 3 separate areas of each flow-chamber experiment were recorded at 1.0-second intervals during 4 minutes. Cells were manually scored according to their rate of interaction with E-selectin/ICAM-1. T lymphoblasts that visibly rolled were defined as “rolling.” Cells which adhered for > 1 second but < 3 seconds and then detached were scored as “transient” attachment and cells adhering > 3 seconds that remained adherent were scored as “firm” attachment. (See supplemental Methods for extra procedures used for cells, immunoprecipitation, Western blotting, confocal and video microscopy, and statistical analysis; available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Image information
Image acquisition data can be found in Supplemental methods.
Results
LFA-1 is associated with the tyrosine kinases Lck and ZAP-70
Using Western blotting, we examined T-lymphoblast lysates for the presence of 7 Src family homologues (Lck, Fyn, Src, Yes, cSrc, ArgSH2/SH3, c-Abl), and the 2 Syk family members, Syk and ZAP-70. Of the Src homologues, Lck and Fyn were detected and ZAP-70 was identified as the major Syk homologue in total T-cell lysates (data not shown).
We then used coimmunoprecipitation to ask whether LFA-1 expressed by human T lymphoblasts and primary T lymphocytes might be associated with these Src and Syk family kinases. When the LFA-1 immunoprecipitate from T blasts in suspension was probed for Lck, Fyn, ZAP-70, and α-tubulin, a positive association was found with Lck and ZAP-70, but not with Fyn or α-tubulin which served as the control for the selectivity of the immunoprecipitation (Figure 1A). Lck and ZAP-70 also coimmunoprecipitated with LFA-1 expressed by freshly isolated primary T lymphocytes in the absence of added stimulant (Figure 1B). These results suggest that LFA-1 is complexed with Lck and ZAP-70 and that the association of both kinases is constitutive and exists in the absence of attachment to ICAM-1 or other stimulants.
LFA-1 is associated with Lck and ZAP-70 in a signaling complex that is activated in response to LFA-1 binding to ICAM-1. (A) Total T-lymphoblast lysate (TL); LFA-1 immunoprecipitates from T lymphoblasts in suspension (IP) blotted for the presence of LFA-1, Lck, ZAP-70, Fyn, and α-tubulin; n = 4. (B) Total primary T-lymphocyte lysate (TL); LFA-1 immunoprecipitates from primary T lymphocytes in suspension (IP) blotted for the presence of LFA-1, Lck, ZAP-70, Fyn, and α-tubulin; n = 3. (C) Lysates of T lymphoblasts either in suspension (Susp) or treated with pan–LFA-1 mAb YTH81.5 and blotted for phospho-Lck (Y394), phospho-ZAP-70 (Y319), and α-tubulin; n = 4. Black vertical lines indicate images taken from separate tracks on the blots. (D) T lymphoblasts were incubated with either ICAM-1– or BSA-coated microspheres for 15 minutes at 37°C. Typical wide field images are shown of the interaction of the T cells (dark images) with the coated microspheres (white spheres). (E) T blasts were incubated for 30 minutes with the microspheres, then lysates were made and blotted for total LFA-1 (control), phospho-Lck (Y394), and phospho-ZAP-70 (Y319). Black vertical lines indicate images taken from separate exposures of the blots. (F) T lymphoblasts either untreated or treated with 40μM PP2 or 10μM piceatannol were incubated on ICAM-1–coated plates. Lysates were probed for phospho-Lck (Y394), total Lck, phospho-ZAP-70 (Y319), total ZAP-70, and α-tubulin by Western blotting; n = 3.
LFA-1 is associated with Lck and ZAP-70 in a signaling complex that is activated in response to LFA-1 binding to ICAM-1. (A) Total T-lymphoblast lysate (TL); LFA-1 immunoprecipitates from T lymphoblasts in suspension (IP) blotted for the presence of LFA-1, Lck, ZAP-70, Fyn, and α-tubulin; n = 4. (B) Total primary T-lymphocyte lysate (TL); LFA-1 immunoprecipitates from primary T lymphocytes in suspension (IP) blotted for the presence of LFA-1, Lck, ZAP-70, Fyn, and α-tubulin; n = 3. (C) Lysates of T lymphoblasts either in suspension (Susp) or treated with pan–LFA-1 mAb YTH81.5 and blotted for phospho-Lck (Y394), phospho-ZAP-70 (Y319), and α-tubulin; n = 4. Black vertical lines indicate images taken from separate tracks on the blots. (D) T lymphoblasts were incubated with either ICAM-1– or BSA-coated microspheres for 15 minutes at 37°C. Typical wide field images are shown of the interaction of the T cells (dark images) with the coated microspheres (white spheres). (E) T blasts were incubated for 30 minutes with the microspheres, then lysates were made and blotted for total LFA-1 (control), phospho-Lck (Y394), and phospho-ZAP-70 (Y319). Black vertical lines indicate images taken from separate exposures of the blots. (F) T lymphoblasts either untreated or treated with 40μM PP2 or 10μM piceatannol were incubated on ICAM-1–coated plates. Lysates were probed for phospho-Lck (Y394), total Lck, phospho-ZAP-70 (Y319), total ZAP-70, and α-tubulin by Western blotting; n = 3.
The next question was whether triggering of LFA-1 had impact on the phosphorylation and activation of these kinases. Our first approach was to cross-link LFA-1 mimicking binding to ligand ICAM-1. T lymphoblasts were untreated or preincubated with the pan–LFA-1 mAb YTH81.5 and investigated for phosphorylation of Lck and ZAP-70. A proportion of Lck was phosphorylated (Y394) in nonadherent T cells, but was further increased after LFA-1 cross-linking (Figure 1C). Active ZAP-70, detected as phospho-ZAP-70 (Y319),36 was minimal in resting nonadherent T blasts, but greatly increased on LFA-1 cross-linking, indicating that ZAP-70 phosphorylation depends on LFA-1 triggering.
The 3-dimensional surfaces of ICAM-1–coated microspheres have been considered to more closely resemble the physiologic display of ligand than 2-dimensional surfaces.37 T blasts became polarized when they interacted with the ICAM-1–coated, but not BSA-coated, microspheres (Figure 1D). Analysis of the phosphorylation status of Lck and ZAP-70 revealed that ICAM-1–microsphere contact enhanced the level of phospho-Lck and induced phospho-ZAP-70 (Figure 1E).
Because Lck and ZAP-70 were both associated with LFA-1, it was logical to investigate the relationship between them. Inhibition of Lck with Src family inhibitor, PP2, blocked both Lck and ZAP-70 phosphorylation in T blasts exposed to ICAM-1. Inhibition of ZAP-70, using Syk/ZAP-70 inhibitor piceatannol, inhibited only ZAP-70 phosphorylation with phospho-Lck levels remaining unchanged (Figure 1F). Thus phosphorylation of ZAP-70 is dependent on the kinase activity of Lck.
Together these data show that LFA-1 on resting primary T cells and T lymphoblasts is complexed with Lck and ZAP-70 tyrosine kinases, and that a hierarchy of action exists between them as ZAP-70 phosphorylation is dependent on active Lck, but not the reverse. Contact with ICAM-1 or cross-linking LFA-1 using mAbs to mimic binding to ICAM-1 is sufficient to induce the phosphorylation and therefore activation of these 2 kinases.
Active ZAP-70 is expressed at the lamellipodia/lamella interface
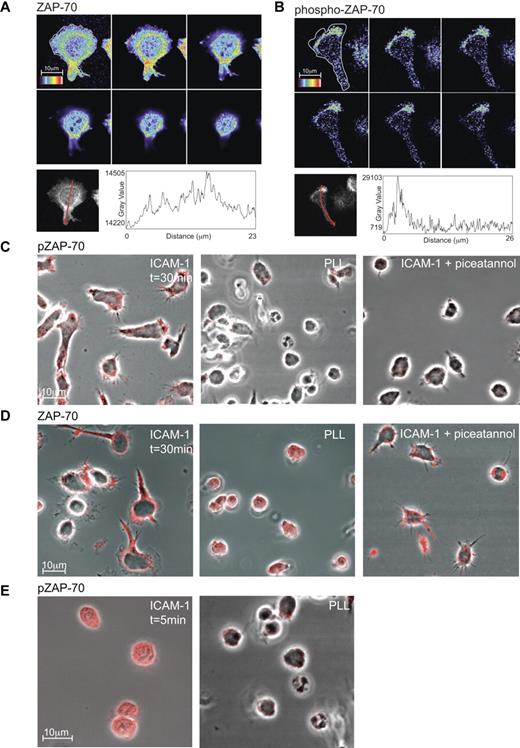
Focusing on ZAP-70, we used confocal microscopy to ask where it functions on the T cell. T blasts migrating for 30 minutes on ICAM-1 were fixed and stained for total ZAP-70 and phospho-ZAP-70 (Y319). Expression of ZAP-70 was generally distributed throughout the cell with some concentration surrounding the nuclear region in a “ring”-like manner and extending toward the attached mid-region of polarized T cells (Figure 2A). In contrast, the highest expression of phospho-ZAP-70 was at the lamellipodia/lamella boundary and closely associated with the interface of T-cell contact with ICAM-1 (Figure 2B). This difference in distribution within the polarized T cell was emphasized when the profiles of z-stacks of ZAP-70 versus phospho-ZAP-70 were compared (Figure 2A-B).
Expression of phospho-ZAP-70 and ZAP-70 in T lymphoblasts. Confocal z-stack (each slice = 0.4 μm) through a typical T lymphoblast polarized on ICAM-1. The distribution of ZAP-70 (A) or phospho-ZAP-70 (Y319; B) is viewed at the interface with ICAM-1 (top left image) and up through the cell. The rainbow scale is applied with white representing the strongest expression. The graphs depict the intensity in expression of ZAP-70 and phospho-ZAP-70 along an arrow across each cell showing where the profile data were collected from the z-stack of T-cell images. (C) Confocal images of T lymphoblasts attached for 30 minutes either to ICAM-1, poly-L-lysine (PLL) or ICAM-1 after treatment with 10μM Syk/ZAP-70 inhibitor piceatannol. The T cells were stained with phospho-ZAP-70 (Y319) mAb and images were taken at the interface with ICAM-1. (D) As in panel C, but the T cells were stained with pan-ZAP-70 mAb. (E) T lymphoblasts exposed to ICAM-1 and to PLL, both for 5 minutes and stained with phospho-ZAP-70 mAb. Images were taken at the interface with ICAM-1. Scale bar, 10 μm.
Expression of phospho-ZAP-70 and ZAP-70 in T lymphoblasts. Confocal z-stack (each slice = 0.4 μm) through a typical T lymphoblast polarized on ICAM-1. The distribution of ZAP-70 (A) or phospho-ZAP-70 (Y319; B) is viewed at the interface with ICAM-1 (top left image) and up through the cell. The rainbow scale is applied with white representing the strongest expression. The graphs depict the intensity in expression of ZAP-70 and phospho-ZAP-70 along an arrow across each cell showing where the profile data were collected from the z-stack of T-cell images. (C) Confocal images of T lymphoblasts attached for 30 minutes either to ICAM-1, poly-L-lysine (PLL) or ICAM-1 after treatment with 10μM Syk/ZAP-70 inhibitor piceatannol. The T cells were stained with phospho-ZAP-70 (Y319) mAb and images were taken at the interface with ICAM-1. (D) As in panel C, but the T cells were stained with pan-ZAP-70 mAb. (E) T lymphoblasts exposed to ICAM-1 and to PLL, both for 5 minutes and stained with phospho-ZAP-70 mAb. Images were taken at the interface with ICAM-1. Scale bar, 10 μm.
Investigating further the conditions necessary for expression of phospho-ZAP-70, we found a dependence on T-cell contact with ICAM-1 (Figure 2C). Specifically, phospho-ZAP-70 was detected when the T cells made contact with ICAM-1 while they were still rounded and remained detectable at the various stages of spreading and elongation (Figure 2C,E). This expression was not induced by contact with poly-L-lysine (PLL) where the T cells notably remained rounded at early or later times of observation (Figure 2C,E). In addition, blocking the activity of ZAP-70 with piceatannol resulted in diminished phospho-ZAP-70 and a failure of the T cells to polarize (Figure 2C). In contrast expression of total ZAP-70 was without dependence on T-cell attachment to ICAM-1, PLL, or exposure to ICAM-1 in the presence of the ZAP-70 inhibitor, piceatannol (Figure 2D).
These findings further confirm the dependence of ZAP-70 phosphorylation on events downstream of LFA-1/ICAM-1 binding with the effect on polarization suggesting an impact on T-cell function.
Active ZAP-70 colocalizes with high-affinity LFA-1 and talin
The binding of the talin FERM domain to the β subunit of integrins increases their affinity for ligand and this also applies to LFA-1.10,38 In our previous work, we established that high-affinity LFA-1 recognized by mAb 24 was associated with talin and expressed in a mid-cell focal zone region of the polarized T cell.10 In this present study, the more rapid fixing of T cells led to the better preservation of membrane, particularly at the leading edge and it was therefore pertinent to reinvestigate the expression of LFA-1 and talin as well as any association with phospho-ZAP-70.
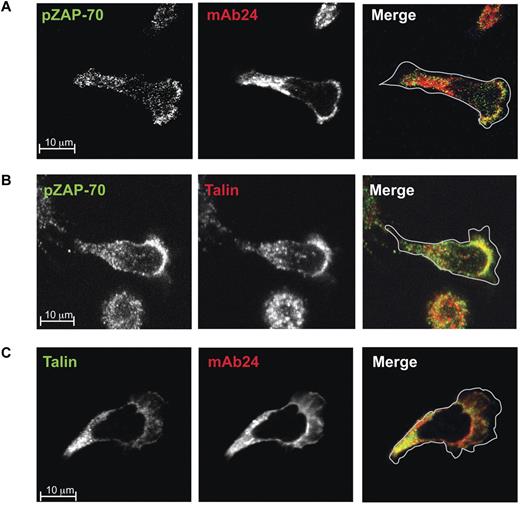
Expression of mAb 24-expressing LFA-1 colocalizing with phospho-ZAP-70 was most evident at the front of the cell at the lamellipodia/lamella interface but also further into the mid-cell lamellar region (Figure 3A). Phospho-ZAP-70 also colocalized with talin with a similar overlap as with + 24 LFA-1 (Figure 3B). It remained to show that + 24 LFA-1 colocalizes with talin at the lamellipodia/lamella interface and even more strongly in the lamellar region (Figure 3C).
Phospho-ZAP-70 colocalizes with high-affinity LFA-1 and talin at the lamellar/lamellipodial interface of migrating T cells. Confocal images of typical T lymphoblasts fixed on ICAM-1 before mAb staining. Images were taken at substrate level and merged images are shown in the right panel. The cells were stained with Alexa 546–conjugated mAb 24 or with mAbs specific for phospho-ZAP-70 and talin which were detected with the Alexa 488 or Alexa 546–conjugated anti–mouse IgG: (A) phospho-ZAP-70 (Y319; green) and mAb 24 (red); (B) phospho-ZAP-70 (Y319; green) and talin (red); (C) talin (green) and mAb 24 (red).
Phospho-ZAP-70 colocalizes with high-affinity LFA-1 and talin at the lamellar/lamellipodial interface of migrating T cells. Confocal images of typical T lymphoblasts fixed on ICAM-1 before mAb staining. Images were taken at substrate level and merged images are shown in the right panel. The cells were stained with Alexa 546–conjugated mAb 24 or with mAbs specific for phospho-ZAP-70 and talin which were detected with the Alexa 488 or Alexa 546–conjugated anti–mouse IgG: (A) phospho-ZAP-70 (Y319; green) and mAb 24 (red); (B) phospho-ZAP-70 (Y319; green) and talin (red); (C) talin (green) and mAb 24 (red).
A pixel-by-pixel determination reinforced the microscopic conclusions that the phospho-ZAP-70 associations with both LFA-1 and talin dominated at the lamellipodia/lamella interface, but also extended further back in the lamellar region (Table 1). The colocalization of mAb 24 and talin was most extensive, implying that this association was able to exist in the absence of phospho-ZAP-70.
In summary, in the polarized T cell, phospho-ZAP-70, colocalizes with high-affinity LFA-1 and talin particularly within a ring at the front of the cell and further back into the lamella. This staining pattern suggests, but does not prove, that a functional relationship exists between phospho-ZAP-70 with LFA-1 and talin.
ZAP-70 activity is necessary for expression of high-affinity LFA-1
As phospho-ZAP-70 colocalized with both LFA-1 and talin, it was possible that ZAP-70 might influence the association of LFA-1 with talin. Alternatively, phospho-ZAP-70 might act downstream of the LFA-1/talin complex. To ask whether the presence of talin was essential for ZAP-70 activity, we made use of the T-cell line HSB2.39 First, we showed that ICAM-1–bound HSB2 cells expressed increased levels of phospho-ZAP-70 compared with cells in suspension similarly to primary T cells (supplemental Figure 1A). Next, we knocked down talin in HSB2 cells using siRNA to reduce its expression by 68%. Previously we showed that ∼ 65% talin knockdown was sufficient to disrupt LFA-1 adhesion.10 However this loss of talin had no effect on the level of phospho-ZAP-70 expressed by ICAM-1–bound T cells (supplemental Figure 1B). Therefore, either ZAP-70 phosphorylation preceded the LFA-1 association with talin or the binding of talin to LFA-1 was independent of ZAP-70 activity. Knocking down α-actinin, a cytoskeletal protein previously shown to colocalize with extended intermediate-affinity LFA-1 in the lamellipodia region of polarized T cells11 also failed to affect ZAP-70 phosphorylation (data not shown).
As reduced talin expression had no impact on ZAP-70 activity, we next asked whether inhibition of ZAP-70 activity altered the expression of high-affinity LFA-1 that is talin-dependent.10 We found that ZAP-70 blockade reduced the expression of high-affinity LFA-1 detected with mAb 24 (Figure 4A), but had no effect on the extended conformation detected using mAb KIM127 (Figure 4B) or total LFA-1 (data not shown). Piceatannol also interfered with polarization as previously noted (Figure 2C-D). Preliminary experiments with ZAP-70 knockdown in HSB2 cells yielded similar results (data not shown).
Inhibition of ZAP-70 prevents expression of high-affinity LFA-1 on T cells migrating on ICAM-1. (A) T lymphobasts were untreated or treated with 10μM piceatannol and stained with high-affinity LFA-1 mAb 24 conjugated to Alexa 546. The mean fluorescence intensity (MFI) was quantified from 3 independent experiments. (B) T lymphobasts were untreated or treated with 10μM piceatannol and stained with mAb KIM127 conjugated to Alexa 546 that detects extended β2 integrin. The MFI was quantified from 3 independent experiments. IRM images of typical T lymphoblasts after 20 minutes on ICAM-1: (C) untreated and (D) after piceatannol treatment. The images of close contact (dark regions) were taken at 20 nm from the substrate surface. Results are representative of n = 4 experiments.
Inhibition of ZAP-70 prevents expression of high-affinity LFA-1 on T cells migrating on ICAM-1. (A) T lymphobasts were untreated or treated with 10μM piceatannol and stained with high-affinity LFA-1 mAb 24 conjugated to Alexa 546. The mean fluorescence intensity (MFI) was quantified from 3 independent experiments. (B) T lymphobasts were untreated or treated with 10μM piceatannol and stained with mAb KIM127 conjugated to Alexa 546 that detects extended β2 integrin. The MFI was quantified from 3 independent experiments. IRM images of typical T lymphoblasts after 20 minutes on ICAM-1: (C) untreated and (D) after piceatannol treatment. The images of close contact (dark regions) were taken at 20 nm from the substrate surface. Results are representative of n = 4 experiments.
We next used interference reflection microscopy (IRM) to gain further information about where ZAP-70 was acting by examining in more detail the contacts made by LFA-1 when T cells encounter ICAM-1 with or without ZAP-70 blockade. Tightly adhering areas of the cell within 20 nm of the ICAM-1 surface are detected as regions of dark contrast in IRM. Untreated T cells were spread and displayed intermittent contact at the cell edges with a darker more sustained contact area corresponding to the lamellar region as observed previously10,11 (Figure 4C; mean spread area of 10 cells = 65.5 ± 5.0 μm). In contrast, in the piceatannol-treated T-cell samples, there was an absence of spread and a 3.5-fold reduction in area of close contact (mean spread area of 10 cells = 18.6 ± 1.2 μm; Figure 4D). The mean area of the cells was mirrored by the mean area of close contacts (untreated: 18.3 ± 1.3 μm; piceatannol-treated: 4.5 ± 0.4 μm). This provided more evidence that active ZAP-70 has an essential role in formation or stabilization of the firm contacts with ICAM-1.
Together confocal and IRM findings indicate that active ZAP-70 has an essential role in the transition of extended intermediate-affinity LFA-1 to the high-affinity LFA-1 conformation that generates the close contacts with ICAM-1 upstream of the signals necessary for cell spreading.
Role of ZAP-70 under shear-flow conditions
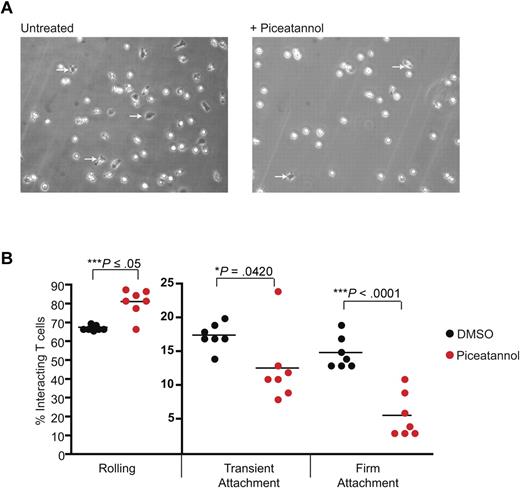
When T cells are subject to shear flow, they briefly roll along a stimulated endothelial surface in a selectin-dependent manner before LFA-1 activation converts short-lived contacts with ICAM-1 to those of firm adhesion.14 It was important to investigate whether ZAP-70 played a role in such LFA-1–mediated events. T blasts were perfused through chambers coated with E-selectin and ICAM-1 at a shear-flow rate of 1 dyne/cm2 with or without piceatannol (Figure 5A). Using these conditions 67% ± 1% of T cells rolled along the E-selectin/ICAM-1 substrate, 17% ± 1% transiently adhered and 15% ± 1% stably adhered (Figure 5B). After treatment with piceatannol, there was a 20% increase in T cells that rolled (81% ± 3%), a 30% decrease in transient adhesion (12% ± 2%) and a 67% decrease in firm adhesion (5% ± 1%). Thus, ZAP-70 function is important where T-cell rolling is converted from transient to firm attachment to ligand ICAM-1.
ZAP-70 is necessary for attachment of T cells under shear flow. (A) T lymphoblasts were untreated or treated with 10μM piceatannol and added to Ibidi chambers coated with 3 μg/mL ICAM-1 and 10 μg/mL E-selectin under a shear flow of 2 dyne/cm2. Firmly adherent cells, all of which were migrating, are identified by dark phase images (arrows). (B) The T cells were categorized as rolling, transiently attached, or firmly attached and the interactions were quantified as a percentage of total interacting cells. The data, illustrated as dots, represent individual 3-minute videos pooled from 3 independent experiments.
ZAP-70 is necessary for attachment of T cells under shear flow. (A) T lymphoblasts were untreated or treated with 10μM piceatannol and added to Ibidi chambers coated with 3 μg/mL ICAM-1 and 10 μg/mL E-selectin under a shear flow of 2 dyne/cm2. Firmly adherent cells, all of which were migrating, are identified by dark phase images (arrows). (B) The T cells were categorized as rolling, transiently attached, or firmly attached and the interactions were quantified as a percentage of total interacting cells. The data, illustrated as dots, represent individual 3-minute videos pooled from 3 independent experiments.
The LFA-1/Lck/ZAP-70 complex is required for LFA-1–mediated T-cell migration
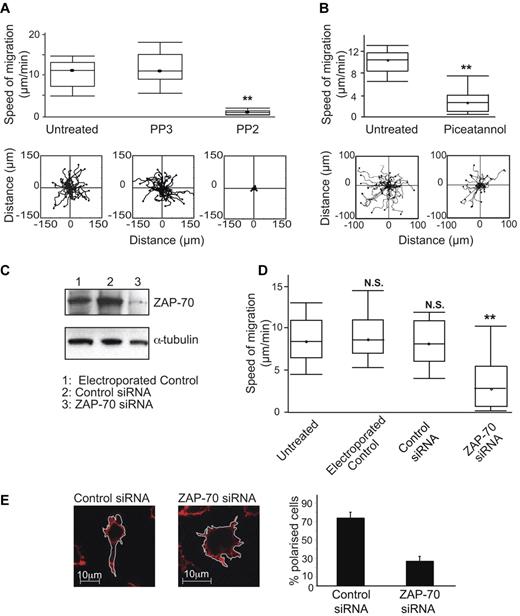
For platelets, macrophages and neutrophils, it is well documented that the Src/Syk kinases function in cell spreading on integrin ligands, but it has been less certain that their activity leads to migration.40,41 To investigate any role for Lck and ZAP-70 in T-cell migration, we first tested the effects of their inhibitors. T cells treated with PP2 had reduced migration rates compared with either untreated or negative control PP3-treated T cells (0.7 ± 0.1 μm/min PP2 vs 12.2 ± 0.8 μm/min control PP3 or 10.8 ± 0.6 μm/min untreated; Figure 6A). Similarly for ZAP-70, treatment with piceatannol reduced the speed of migration on ICAM-1 compared with untreated T cells (3.7 ± 0.7 μm/min piceatannol vs 9.3 ± 0.4 μm/min untreated; Figure 6B).
Lck and ZAP-70 are both necessary for T-cell migration on ICAM-1. (A) T lymphoblasts were either untreated or treated with 40μM Src family inhibitor PP2 or control PP3 and incubated on immobilized ICAM-1 before observation for 20 minutes by video microscopy and the migration of individual cells tracked. A boxplot (box-and-whisker plot) of 3 independent migration experiments is shown in the top panel and a representative experiment showing the migratory tracks of individual cells from each treatment condition in the bottom panel; n = 40 cells per condition. (B) T lymphoblasts were either untreated or treated with 10μM Syk family inhibitor piceatannol and processed as in panel A. (C) HSB2 T cells were electroporated without siRNA as a control, with 400nM control siRNA or ZAP-70 siRNA. Western blots were probed for total ZAP-70 and α-tubulin served as a loading control. (D) HSB2 T cells that were either untreated, electroporated without siRNA, with control siRNA or with ZAP-70 siRNA were observed on ICAM-1 by video microscopy for 20 minutes. A boxplot of 3 independent migration experiments is shown. (E) Confocal images of typical HSB2 T cells transfected with control siRNA or with ZAP-70 siRNA on ICAM-1. The T cells are stained for actin with phalloidin–Alexa 546. Quantification of the proportion of polarized T cells after siRNA transfection: 74%, control; 27%, ZAP-70 knockdown; n = 105 cells for each condition.
Lck and ZAP-70 are both necessary for T-cell migration on ICAM-1. (A) T lymphoblasts were either untreated or treated with 40μM Src family inhibitor PP2 or control PP3 and incubated on immobilized ICAM-1 before observation for 20 minutes by video microscopy and the migration of individual cells tracked. A boxplot (box-and-whisker plot) of 3 independent migration experiments is shown in the top panel and a representative experiment showing the migratory tracks of individual cells from each treatment condition in the bottom panel; n = 40 cells per condition. (B) T lymphoblasts were either untreated or treated with 10μM Syk family inhibitor piceatannol and processed as in panel A. (C) HSB2 T cells were electroporated without siRNA as a control, with 400nM control siRNA or ZAP-70 siRNA. Western blots were probed for total ZAP-70 and α-tubulin served as a loading control. (D) HSB2 T cells that were either untreated, electroporated without siRNA, with control siRNA or with ZAP-70 siRNA were observed on ICAM-1 by video microscopy for 20 minutes. A boxplot of 3 independent migration experiments is shown. (E) Confocal images of typical HSB2 T cells transfected with control siRNA or with ZAP-70 siRNA on ICAM-1. The T cells are stained for actin with phalloidin–Alexa 546. Quantification of the proportion of polarized T cells after siRNA transfection: 74%, control; 27%, ZAP-70 knockdown; n = 105 cells for each condition.
To further confirm the effects on migration of ZAP-70 inhibition, we used siRNA to reduce ZAP-70 expression in HSB2 T cells by 81% without an adverse effect on viability (Figure 6C). These cells had significantly decreased speed of migration on ICAM-1 compared with control cells (3.8 ± 0.4 μm/min ZAP-70 siRNA vs 8.4 ± 0.4 μm/min control siRNA; Figure 6D), replicating the effects of piceatannol.
Confocal microscopy (Figure 6E) and live imaging of HSB2 cells after treatment with control siRNA (supplemental Video 1) or ZAP-70 siRNA (supplemental Video 2) showed that when ZAP-70 was deficient, the cells were attached, but lacked polarity and motility and displayed dynamic membrane protrusions in all directions compared with control T cells. These effects of ZAP-70 knockdown mimicked the effects of piceatannol (Figures 2C-D, 4C-D), providing more evidence for a connection between ZAP-70 and LFA-1 elongation leading to migration. Finally, siRNA knockdown of Lck by 83% (data not shown) in T cells also significantly decreased the speed of LFA-1-mediated migration (supplemental Figure 2). Therefore, in terms of function, the protein tyrosine kinases Lck and ZAP-70 have an essential role in the migration of T lymphocytes.
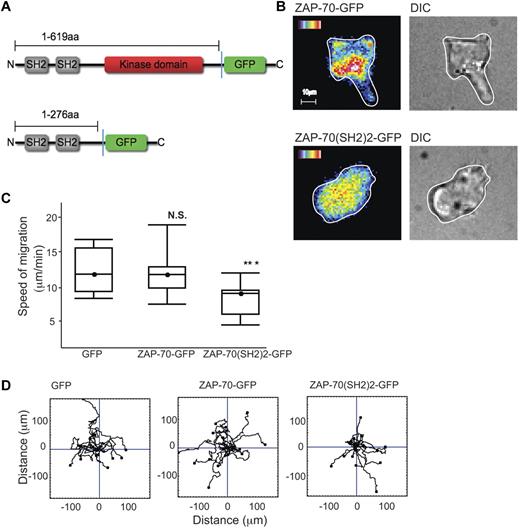
ZAP-70–GFP and LFA-1–mediated migration
To observe how the distribution of ZAP-70 might alter dynamically as the T-cell migrates, we transfected HSB2 T cells with a ZAP-70-GFP cDNA fusion construct or truncated GFP-tagged ZAP-70 cDNA containing only the 2 SH2 domains (ZAP-70(SH2)2-GFP) (Figure 7A).42 As the ZAP-70–GFP+ T cells migrated on ICAM-1, ZAP-70 was observed just behind the leading edge with an elevated level visible further back in the T cell as in Figure 2A (Figure 7B). During migration, the leading edge ZAP-70 dynamically altered in correlation with the direction of migration (supplemental Video 3). In contrast, in the ZAP-70(SH2)2-GFP–expressing cells, the fluorescence was generally distributed within the T cells which were rounded and lacked motility (Figure 7B). These findings suggested that ZAP-70(SH2)2-GFP lacking the kinase domain was interfering with endogenous ZAP-70 function.
ZAP-70-GFP, ZAP-70(SH2)2-GFP and LFA-1–mediated migration. (A) Schematic of ZAP-70-GFP and ZAP-70(SH2)2-GFP proteins encoded by cDNA constructs used in this study. (B) HSB2 T cells transfected with either ZAP-70-GFP or ZAP-70(SH2)2-GFP cDNAs by electroporation and allowed after 24 hours to migrate on ICAM-1; representative of n = 2 experiments per treatment. (C) HSB2 T cells that were electroporated with either GFP, ZAP-70-GFP, or ZAP-70(SH2)2-GFP cDNA constructs were allowed to migrate on ICAM-1 for 20 minutes. A boxplot of 3 independent migration experiments is shown and in panel D, a representative experiment of the migratory tracks of individual cells of each transfectant; n = 40 cells per condition.
ZAP-70-GFP, ZAP-70(SH2)2-GFP and LFA-1–mediated migration. (A) Schematic of ZAP-70-GFP and ZAP-70(SH2)2-GFP proteins encoded by cDNA constructs used in this study. (B) HSB2 T cells transfected with either ZAP-70-GFP or ZAP-70(SH2)2-GFP cDNAs by electroporation and allowed after 24 hours to migrate on ICAM-1; representative of n = 2 experiments per treatment. (C) HSB2 T cells that were electroporated with either GFP, ZAP-70-GFP, or ZAP-70(SH2)2-GFP cDNA constructs were allowed to migrate on ICAM-1 for 20 minutes. A boxplot of 3 independent migration experiments is shown and in panel D, a representative experiment of the migratory tracks of individual cells of each transfectant; n = 40 cells per condition.
To better quantify any difference in behavior of the 2 constructs and a control GFP construct, HSB2 T cells were transfected and selected for equivalent GFP expression by FACS sorting. HSB2 T cells transfected with the ZAP-70(SH2)2-GFP cDNA had a significantly reduced rate of migration (mean ± SEM for T cells transfected with control GFP, 12 ± 1.0 μm/min; with ZAP-70-GFP, 12.3 ± 1.2 μm/min; compared with ZAP-70(SH2)2-GFP, 8.9 ± 0.8 μm/min; Figure 7C). The cell-tracking data confirmed that ZAP-70(SH2)2-GFP cDNA did not alter the ability of the cells to migrate randomly (Figure 7D).
Therefore, ZAP-70 is enriched at the leading edge of the migrating T cell and the ability of a ZAP-70 construct expressing only the SH2 domains to act as a dominant inhibitor implicates the SH2 domains in the ZAP-70 activation that drives T-cell migration.
Discussion
In this study, we have explored the signaling activity of the β2 integrin LFA-1 expressed on human T lymphocytes by characterizing its associated kinases. We find that the Src family kinase Lck and Syk family kinase ZAP-70 form a signaling complex with LFA-1 and this complex is most highly concentrated in the lamella of T lymphocytes at the interface with ICAM-1. The kinases become phosphorylated after LFA-1 binding to ICAM-1 and therefore constitute early mediators of outside-in signaling. They are essential for conversion of intermediate- to high-affinity LFA-1 and for the firm adhesion and migration of T cells on ICAM-1 under both static and shear-flow conditions.
Coimmunoprecipitation experiments show that LFA-1 complexes with both Lck and ZAP-70. The fact that this association is detected in unstimulated primary T lymphocytes as well as T lymphoblasts implies that it is constitutive and not a result of T-cell activation. In the well-described interaction of Src and the ZAP-70 homologue, Syk with platelet integrin αIIbβ3, Src is constitutively associated with αIIbβ3, but Syk becomes integrin–bound only after platelet adhesion to fibrinogen.22 Similarly, Syk associates with β2 integrin in neutrophils only after adhesion to ligand.20 Thus the fact that ZAP-70 is constitutively associated with LFA-1 in T lymphocytes distinguishes it from the induced association of its homologue Syk with integrins in other primary leukocytes.
The antigen-specific TCR complex also signals through both Lck and ZAP-70 suggesting that LFA-1 might be part of the same multimolecular complex.31,32 However, neither coimmunoprecipitation nor confocal experiments detected any association of LFA-1 with TCR in unstimulated T cells (data not shown). Moreover, the HSB2 T-cell line used in this study does not express the standard γδ or αβ TCR/CD3 complexes at the cell surface (Hara et al39 ; data not shown). Further support for the independence of the LFA-1 complexes comes from a study showing that microclusters of TCR and LFA-1 form at the T-cell periphery in a segregated manner and move separately toward the immunologic synapse.43
For myeloid cells and platelets, “outside-in” signaling involves the Src family kinase-mediated phosphorylation of ITAM motifs present on accessory proteins.26,27,30 The phospho-ITAMs in turn provide docking sites for the dual SH2 domains of Syk. For T cells, the activation of ZAP-70 downstream of the TCR strictly requires binding via its SH2 domains to ITAMs on the CD3 and ζ proteins. We provide evidence that ZAP-70 function in T-cell migration involves ZAP-70 SH2 domains, potentially binding to ITAMs, as a ZAP-70 construct consisting of tandem SH2 domains acts as an inhibitor of T-cell migration. ZAP-70 phosphorylation is blocked under these circumstances (A.C.L., unpublished observations, June 2009). Previous reports show that Syk and ZAP-70 can use their N-terminal SH2 and interdomain region A domains to bind directly to the integrin β subunit indicating the exact role of the SH2 domains needs to be further explored.28,29 However, it is plausible that a separate ITAM-containing adapter molecule may also be part of this LFA-1 signaling complex, adding to the accumulating evidence of the similarity of integrin signaling with the early steps of immunoreceptor signaling.44
T-cell contact with ICAM-1 or cross-linking of LFA-1 induces phosphorylation of ZAP-70 at Y319 in the SH2-kinase “B” linker and Y493 in the kinase domain and thus its activation. In contrast, a proportion of LFA-1–associated Lck is phosphorylated before ICAM-1 binding, with subsequent binding enhancing the levels of phospho-Lck. While it is possible that manipulation involved in T-lymphocyte isolation may give sufficient stimulus to generate low levels of phospho-Lck, Acuto and colleagues have demonstrated that a proportion of total Lck is in a TCR-independent, constitutively active form in unstimulated naive T cells and thymocytes.31,45 The phospho-Lck associated with LFA-1 may be part of this pool of active kinase and it is of interest to consider how its activity might be regulated when LFA-1–associated and how it avoids phosphorylating any nearby ITAM-expressing proteins. There is evidence that the TCR-associated Lck phosphorylation is dynamically regulated by the phosphatase CD45. There is also evidence that CD45 can bind to LFA-146 suggesting a similar method of phosphorylation control. A conformational change in LFA-1 after binding to ICAM-1 may then orchestrate an alteration in the relationship between Lck/ZAP-70 and potentially ITAM-containing proteins that would allow the activating phosphorylations to proceed. Further advances in understanding the composition of the LFA-1–centred signaling complex await proteomic analysis.
Inside-out signaling either through chemokine receptors on lymphocytes or E-selectin–induced signaling through its ligand PSGL1 on neutrophils results in the extension of LFA-1 and presentation of an intermediate conformation.47,48 Here, we are describing an outside-in signaling step initiated by the extended intermediate conformation of LFA-1 expressed on T cells. Thus LFA-1 binding to ICAM-1 is sufficient to cause ZAP-70 phosphorylation and, conversely, blocking ZAP-70 function prevents the conversion of intermediate to high-affinity LFA-1.
A central question is how ZAP-70 brings about this conversion. Confocal images revealed total ZAP-70 to have highest distribution in the lamellar region that is mirrored by a ZAP-70–GFP fusion construct expressed in migrating T cells, whereas phospho-ZAP-70 is most concentrated in the region where the lamella interfaces with lamellipodium. A pixel-by-pixel evaluation reveals that the association of phospho-ZAP-70 overlapped both 24 epitope + LFA-1 and talin at the lamellar interface and also extends throughout the lamella. The associations suggest the existence of a functional relationship between these 3 molecules.
Talin binding to the β subunit is associated with the high-affinity conformation of integrins, including LFA-1.10,38 However, the lack of effect of talin knockdown on phospho-ZAP-70 levels indicates that the LFA-1/talin interaction follows ZAP-70 activation. The recent findings of Garcia-Bernal and colleagues suggest further molecular detail by showing that in T cells, talin in complex with Vav1 is already associated with integrin α4β1. However, this talin is unable to activate integrin until dissociation of talin/Vav1 that is a result of Vav1 phosphorylation by ZAP-70.49 Preliminary evidence suggests that Vav1 is also a ZAP-70 target when T cells are migrating on ICAM-1 (A.M., unpublished data, May 2010). As overlap of LFA-1 and talin exceeds the association of either protein with phospho-ZAP-70, it seems probable that once LFA-1/talin complexes are formed, they are stable without further interaction with ZAP-70.
In terms of T-cell function, reduction in Lck and ZAP-70 expression has a dramatic effect on adhesion and migration that is consistent with the diminished levels of high-affinity LFA-1.10,11 The lack of high-affinity LFA-1 also affects the behavior of T cells when exposed to shear-flow conditions. In the absence of ZAP-70, the conversion from transient to firmer ICAM-1 attachments is prevented. This finding is in keeping with previous reports that have linked the firm attachment of T cells under flow conditions to high-affinity LFA-1.12 Together, the coimmunoprecipitation, confocal, and IRM data indicate that the binding of intermediate-affinity LFA-1 to ICAM-1 initiates ZAP-70 activation and this event results in expression of the high-affinity LFA-1 conformation that controls the stable contacts formed by T cells in contact with ICAM-1.
In summary, we find that an LFA-1/Lck/ZAP-70 signaling complex is essential for adhesion and migration of T cells. This is in keeping with earlier work showing involvement of ZAP-70 in LFA-1–mediated penetration of fibroblast monolayer by a mouse T-cell hybridoma.50 The control of high-affinity conformation of LFA-1 by phospho-Lck and ZAP-70 that are already in complex with the integrin helps to explain how arrest under flow occurs with subsecond timing.6,12 The LFA-1 complexes, containing Lck, ZAP-70, and potentially other proteins such as talin and ITAM-expressing proteins occur predominantly at the lamellar interface of the polarized T cell. This region is therefore equipped to initiate rapid, stable contact with ICAM-1 and activation of subsequent signaling events that promote cell spreading and motility. Such fast action would have particular relevance during an ongoing immune response when it is essential for T cells to respond rapidly to stimulated vasculature at sites of infection or injury.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the generous help of Dr Daniel Zicha (Cancer Research UK LRI) with the IRM experiments and computation, and Leukocyte Adhesion Laboratory colleagues for their comments on this manuscript.
L.S. was supported by a Marie Curie individual fellowship. This work was funded by Cancer Research UK, in part by a Royal Society/CNRS International Joint Project award (N.H. and A.C.L.) and the Association pour la Recherche sur la Cancer (A.C.L.).
Authorship
Contribution: R.E. performed experiments, interpreted results, and assisted in writing the manuscript; A.C.L., L.S., and A.M. performed experiments and commented on the manuscript; and N.H. directed the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Nancy Hogg, 1 Leukocyte Adhesion Laboratory, Cancer Research UK London Research Institute, London WC2A 3PX, United Kingdom; e-mail: nancy.hogg@cancer.org.uk.