Abstract
Recently, a growing body of evidence has suggested that adiponectin, which is secreted by adipose tissues, plays a critical role in obesity-related and autoimmune diseases. We compared the concentrations of adiponectin among 26 normal subjects and 34 allogeneic stem cell transplantation recipients. The concentrations of adiponectin were significantly higher in recipients with chronic graft-versus-host disease (cGVHD) than those in subjects without cGVHD (21.7 ± 11.0 vs 9.1 ± 6.1 μg/mL in females, P < .001; and 10.1 ± 6.8 vs 4.3 ± 2.9 μg/mL in males, P = .003). Multivariate analysis revealed that a higher concentration of adiponectin was associated with female sex (β-coefficient 8.2, P < .0001) and the severity of cGVHD (β-coefficient 6.6, 12.7, and 15.6, P < .01, each for mild, moderate, and severe cGVHD, respectively). In addition, adiponectin levels increased as cGVHD progressed, decreased as cGVHD improved, and did not change with stable cGVHD. In conclusion, adiponectin was associated with the severity of cGVHD and might play a role in the pathophysiology of cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is a major problem after allogeneic stem cell transplantation (allo-SCT) and dramatically impairs the recipient's quality of life.1,2 Clinical symptoms of cGVHD resemble those of autoimmune diseases, including scleroderma and sicca syndrome. Therefore, an immune abnormality similar to that in autoimmune diseases may play a role in the development of cGVHD, although the actual pathophysiology remains unknown.3 In cGVHD, inflammatory cytokines that modulate T and B cells, such as tumor necrosis factor-α and soluble B-cell activation factor, have been well investigated.4,5 However, it remains to be elucidated whether other endocrine substances may play a role in cGVHD. Recently, it has been revealed that adiponectin, an adipokine that is secreted by adipose tissues, is associated with immunity and inflammation and may play a critical role in both obesity-related and autoimmune diseases.6-11 Therefore, we compared the serum adiponectin levels among allo-SCT recipients and normal subjects to assess the association between adiponectin and cGVHD.
Methods
We retrospectively reviewed the clinical records of 34 patients (21 with myeloid malignancies, 11 with lymphoid malignancies, and 2 with aplastic anemia) who received allo-SCT between March 2008 and March 2010 and who survived for at least 180 days after SCT. The diagnosis and the severity of cGVHD were determined based on the National Institutes of Health classification.12 In addition, we collected data regarding age, sex, and body mass index (BMI) of these patients as well as those of 26 healthy subjects as a control.
We measured the serum concentrations of high-molecular-weight (HMW) adiponectin by an enzyme-linked immunosorbent assay according to the manufacturer's instructions (Fujirebio Inc). To compare HMW-adiponectin levels among groups, we used the first samples obtained at least 6 months after SCT and performed Student t test/analysis of variance for categorical variables and a regression analysis for continuous variables. Thereafter, multiple regression analysis was performed with backward stepwise selection. Furthermore, we calculated the ratios of later-to-prior HMW adiponectin between each pair of consecutive samples in the same patients and assessed the impact of the clinical changes in cGVHD on HMW adiponectin concentrations using analysis of variance after logarithmic conversion among recipients grouped according to worsening, stable, and improving cGVHD. Statistical significance was defined as a 2-sided P value of less than .05. This study was approved by the Institutional Review Board of Jichi Medical University, and all patients gave written informed consent for the cryopreservation and analyses of blood samples in accordance with the Declaration of Helsinki.
Results and discussion
Patient characteristics
The characteristics of the 34 allo-SCT recipients are presented in Table 1. Of these, 22 had cGVHD at the sampling, including 7 and 4 with moderate and severe cGVHD, respectively. Sampling was performed at a median 189 days after SCT (range, 177-434 days). Their median age was 38.5 years (range, 16-65 years). Their median weight loss after SCT and median BMI at sampling were 4.1 kg (range, −16.9-26.5 kg) and 20.3 kg/m2 (range, 15.3-34.6 kg/m2), respectively. The 26 normal healthy subjects included 11 males and 15 females. Their median age and BMI were 40 years (range, 28-68 years) and 21.71 kg/m2 (range, 17.31-29.47 kg/m2), respectively.
Comparison of the concentrations of HMW adiponectin
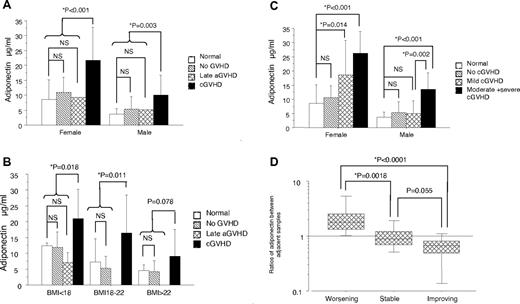
Sex and BMI are known to be associated with the concentration of adiponectin.13 Therefore, HMW adiponectin levels were compared among groups stratified according to sex and BMI (Figure 1A-B). The concentrations of HMW adiponectin were significantly higher in recipients with cGVHD than those in subjects without cGVHD (21.7 ± 11.0 vs 9.1 ± 6.1 μg/mL in females, P < .001; and 10.1 ± 6.8 vs 4.3 ± 2.9 μg/mL in males, P = .003), respectively (Figure 1A). The concentration of HMW adiponectin was inversely correlated with BMI (R2 = 0.20, P = .0003). Furthermore, the HMW adiponectin levels in recipients with cGVHD were higher than those in normal subjects and recipients without cGVHD in each stratified BMI group (Figure 1B).
The levels and kinetics of HMW adiponectin. (A) Concentrations of HMW adiponectin by sex among normal healthy subjects and recipients without GVHD, with late acute GVHD, and with National Institutes of Health cGVHD. (B) Concentrations of HMW adiponectin among normal health subjects and recipients without GVHD, with late acute GVHD, and with National Institutes of Health cGVHD according to BMI less than 18, 18 to 22, and more than 22 kg/m2 groups. (C) Concentrations of HMW adiponectin by sex and the NIH severity of cGVHD. (D) Comparison of the ratios of the later-to-prior HMW adiponectin between adjacent samples according to clinical changes in cGVHD: worsening, improving, and stable cGVHD groups. Ratio > 1 indicates that adiponectin levels increased over time; ratio = 1, adiponectin levels did not change over time; and ratio < 1, adiponectin levels decreased over time.
The levels and kinetics of HMW adiponectin. (A) Concentrations of HMW adiponectin by sex among normal healthy subjects and recipients without GVHD, with late acute GVHD, and with National Institutes of Health cGVHD. (B) Concentrations of HMW adiponectin among normal health subjects and recipients without GVHD, with late acute GVHD, and with National Institutes of Health cGVHD according to BMI less than 18, 18 to 22, and more than 22 kg/m2 groups. (C) Concentrations of HMW adiponectin by sex and the NIH severity of cGVHD. (D) Comparison of the ratios of the later-to-prior HMW adiponectin between adjacent samples according to clinical changes in cGVHD: worsening, improving, and stable cGVHD groups. Ratio > 1 indicates that adiponectin levels increased over time; ratio = 1, adiponectin levels did not change over time; and ratio < 1, adiponectin levels decreased over time.
When we analyzed only allo-SCT recipients, a univariate analysis revealed that a high HMW adiponectin level was associated with female sex, low BMI, the presence and the severity of cGVHD, gut involvement, and steroid administrations. Multiple regression analysis revealed that a high HMW adiponectin level was associated with female sex, steroid administrations, and the severity of cGVHD among allo-SCT recipients (Table 1). When the 26 normal subjects were included, it was further confirmed that a high HMW adiponectin level was associated with female sex (β-coefficient = 8.2; 95% confidence interval, 4.7-11.8, P < .0001) and the severity of cGVHD (β-coefficient = 6.6; 95% confidence interval, 1.8-11.4; β-coefficient = 12.7; 95% confidence interval, 7.0-18.3; and β-coefficient = 15.6; 95% confidence interval, 8.4-22.9, P < .01 each for mild, moderate, and severe cGVHD, respectively), whereas there was no difference between recipients without cGVHD and normal subjects (P = .28; Figure 1C).
Impact of clinical changes in cGVHD on HMW adiponectin
To assess the impact of clinical changes in cGVHD on serum HMW adiponectin levels, we analyzed 58 serial sera with a median interval of 84 days (range, 24-196 days) from 19 patients. Worsening, improving, and stable cGVHD were observed in the 13, 7, and 19 sample pairs, respectively. The ratios of the later-to-prior HMW adiponectin levels between adjacent samples were associated with clinical changes in cGVHD; the HMW adiponectin level increased as cGVHD progressed, decreased as cGVHD improved, and did not change with stable cGVHD (Figure 1D, P < .01 each for comparison between worsening and stable/improving pairs).
Adiponectin is a type of adipokine, which are peptides secreted mainly from adipose tissues. It exists in multimers, and HMW adiponectin is thought to have the greatest effect on immunity and inflammation.7 However, adiponectin has been shown to have both proinflammatory and anti-inflammatory functions; thus, it is difficult to interpret the relationship between a high serum adiponectin level and the development of cGVHD.
First, adiponectin itself might induce cGVHD via its proinflammatory effect. In rheumatoid arthritis models, adiponectin stimulated the secretion of proinflammatory cytokines, including interleukin-6, interleukin-8, matrix metalloproteinase, and monocyte chemoattractant protein-1.6,8,14 In addition, adiponectin has been reported to stimulate the production of extracellular matrix by dermal fibroblasts.15 Therefore, adiponectin may directly induce excessive extracellular matrix and fibrosis as a symptom of cGVHD.
On the other hand, the high adiponectin level in patients with cGVHD might be a response to inflammation in cGVHD because adiponectin has been shown to have an anti-inflammatory function. In vitro, adiponectin suppressed proinflammatory cytokines, including tumor necrosis factor-α, interleukin-1β, and adhesion molecule activities in human endothelial and cardiac cells.16-18 Indeed, several clinical observations have suggested that a high adiponectin level may help protect against vascular inflammation in obesity-related diseases, such as type 2 diabetes mellitus, cardiovascular disease, and metabolic syndrome.7,19-22 The adiponectin level may increase to suppress the systemic inflammation of cGVHD as observed in Sjogren syndrome, in which increasing adiponectin rescues salivary gland epithelial cells from apoptosis.23
To date, an Italian group has evaluated the serum adiponectin levels in the context of metabolic syndrome in long-term allo-SCT survivors.24 The mean adiponectin level was 15.8 and 22.6 μg/mL in recipients with and without metabolic syndrome, respectively. The values seemed higher than those in normal subjects. Although they did not analyze the relationship between the serum adiponectin level and cGVHD, a high adiponectin level might also be associated with cGVHD.
To our knowledge, the current study is the first to suggest an association between the serum adiponectin level and the severity of cGVHD. However, there might be a bias because of the retrospective nature of the study and the small population. Therefore, a further, large prospective study is warranted to assess the association between adiponectin and the severity of cGVHD. In addition, a further basic investigation is also needed to elucidate whether a high adiponectin level in cGVHD is a primary or secondary event and how adiponectin influences the pathophysiology of cGVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.N. designed the study, collected and analyzed data, and wrote the manuscript; P.N.T.B., R.Y., Y.T., K.S., M.A., M.S., K.T., S. Kimura, M.K., S. Kako, S.O., K.O., A.T., J.N., and Y.A. collected data; and Y.K. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoshinobu Kanda, Division of Hematology, Saitama Medical Center, Jichi Medical University, 1-847, Amanuma-cho, Omiya-ku, Saitama-shi, Saitama 330-8503, Japan; e-mail: ycanda-tky@umin.ac.jp.