Abstract
The study goal was to characterize older chronic lymphocytic leukemia (CLL) patients and to evaluate outcomes in those patients who initiated infused therapy. Patients 66 years of age and older in the Surveillance, Epidemiology, and End Results (SEER) program with a CLL diagnosis were matched to their Medicare Part A and Part B claims for long-term follow-up. Treatment patterns, survival after initiation of infused therapy, and both hematologic and hospitalization outcomes were assessed. There were 6433 CLL patients identified, and 2040 received infused therapy. Treated patients were categorized as receiving rituximab monotherapy (16%), rituximab plus chemotherapy (14%), and chemotherapy alone (70%) based on the initial 60 days after infusion. Rituximab plus chemotherapy compared with chemotherapy alone was associated with a 25% lower risk of overall mortality (95% confidence interval, 9%-38%). Restricting to patients age 70 years and older did not change the risk reduction for rituximab plus chemotherapy. Hematologic interventions were more common with rituximab plus chemotherapy compared with chemotherapy alone, but there was no difference in all-cause hospitalizations. These analyses, based on observational data, suggest that the benefits of initial therapy with rituximab in a heterogeneous group of older CLL patients are comparable with those demonstrated in younger patients.
Introduction
Chronic lymphocytic leukemia (CLL) is generally a slowly progressive cancer characterized by increasing levels of lymphocytes in the blood, bone marrow, and lymphatic tissues.1 Many patients are managed with periodic observation or “watch and wait.”2 The selection of therapy is based on both the severity of the CLL as well as patient characteristics, including comorbidities. The current National Comprehensive Cancer Network guidelines suggest that frail patients or those with significant comorbidity can often be treated with oral therapy (eg, chlorambucil) or single-agent rituximab.3 The guidelines for other patients depend in part on age and other patient characteristics and include 10 potential regimens. In these guidelines, chemo-immunotherapy (eg, rituximab plus fludarabine and cyclophosphamide [R-FC]) is preferred for patients younger than 70 years. For patients 70 years of age and older, 6 regimens are suggested with none specified as preferred.
Recently, the German CLL Study Group completed the CLL8 study, a randomized, controlled trial of 817 previously untreated CLL patients.4,5 In this study, R-FC was shown to significantly improve progression-free and overall survival compared with FC alone. However, post-hoc exploratory analyses of the CLL8 data showed no benefit of R-FC in the 10% of patients who were 70 years of age or older (n = 81).6
Although the results from clinical trials provide strong evidence of efficacy, evaluations of interventions as they are used in actual clinical practice are also important. Such “comparative effectiveness research” is designed to provide information about the effects of an intervention, both positive and negative, in the population and conditions in which it is actually used. For cancer, this typically requires evaluating older populations with higher levels of comorbidity than are typically seen in trial populations. One of the limitations of this research is that it can take years to accrue the patient numbers and follow-up time to make such evaluations.
Considering that 69% of newly diagnosed CLL patients are Medicare aged (65 years or older)7 and that rituximab has been commercially available since 1998, a significant repository of experience with rituximab already exists and can be reported contemporaneously with the clinical trial results. Accordingly, we set out to address 2 primary aims with these data: (1) to characterize elderly (Medicare-aged) CLL patients, including their initial use of infused therapies; and (2) to evaluate outcomes in those patients initiating infused therapy.
Methods
Data source
We used the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results (SEER) cancer registry linked to Medicare enrollment and claims data (SEER-Medicare data).8 SEER collects and publishes cancer incidence and survival data from 18 population-based cancer registries throughout the United States covering approximately 26% of the US population.9 The registries routinely collect data, including patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status. In the SEER-Medicare data, for persons 65 years of age or older, 97% are eligible for Medicare and 93% of patients in the SEER files are matched to the Medicare enrollment file.10 At the time of our study, the SEER-Medicare linkage included all Medicare-eligible persons appearing in the SEER data through 2005 and their Medicare claims for Part A (inpatient) and Part B (outpatient and physician services) through 2007.
Patient eligibility
Patients were included in this study if their first, primary cancer was diagnosed as CLL between January 1, 1999, and December 31, 2005. Identification of CLL was based on site code 75 in the SEER data. Patients were excluded for the following reasons: diagnosis in the month of death, Medicare enrollment less than 12 months before diagnosis, or at least 2 Medicare claims for chemotherapy treatment before diagnosis. In addition, to ensure complete claims history, patients had to have been enrolled in both Medicare Parts A and B, with no health maintenance organization coverage for 12 months before diagnosis (making the minimum age in the cohort 66 years). After diagnosis, patients were followed until death, enrollment in a health maintenance organization, development of a second primary tumor, or the last date for which Medicare claims were available. Supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) contain a schematic of the inclusion/exclusion process.
Treatments
Medicare claims were used to identify the date of the first infused therapy provided to patients after diagnosis, using International Classification of Diseases, 9th Revision, Clinical Modification procedure codes11 and Healthcare Common Procedure Coding System codes.12 Oral therapies without an intravenous equivalent (eg, chlorambucil) are not reimbursed by Medicare Part B and are not available in the data. Patients who received infused therapy were classified into one of 3 groups based on claims from the first 60 days after the initial infusion: chemotherapy alone, rituximab therapy alone, or rituximab plus chemotherapy.
It is important to note that not all chemotherapy claims had a code to indicate which specific drugs were used. As a result, we further classified chemotherapy by the use of fludarabine, cyclophosphamide, and all other therapies. The term “other” reflects the use of chemotherapy identified either using Healthcare Common Procedure Coding System codes (ie, J-codes) for the specific agent or using diagnosis or procedure codes indicating chemotherapy use without a J-code.13 Additional classification was not practical given the nature of medical claims data.
Mortality and censoring
The date of death was assigned using the Medicare date, unless it was missing, in which case the SEER date of death was used. All other patients were assumed to be alive at the end of the analysis period (December 31, 2007), although they may have been censored earlier for other reasons described under “Patients eligibility.”
Patient characteristics
Patients were described according to their demographic, clinical, and socioeconomic characteristics. Patient age was stratified into 4 groups (66-69, 70-74, 75-79, and ≥ 80 years) and was defined either at diagnosis or at the initiation of infused therapy, as appropriate for specific analyses. Requiring eligible patients to have at least one year of Medicare enrollment before diagnosis ensured that the minimum age in the cohort was 66 years. Race/ethnicity was defined using the SEER recoded race variable as white, black, Hispanic, and other (which consists predominantly of Native American/Native Alaskan, Native Hawaiian or Other Pacific Islander, and Asian).14
Stage is not available for leukemia in the SEER data.15 Because the available staging systems assign patients with anemia or thrombocytopenia to more advanced stages, we used the presence of these diagnoses in the claims to classify patients as “advanced stage” for our analyses (see “Statistical analysis”). We used the Medicare inpatient (Part A) and physician/outpatient facility (Part B) claims to calculate a National Cancer Institute comorbidity index for each patient.16 This approach involves first removing claims that are considered to have unreliable diagnosis coding, such as those for testing procedures used to rule out conditions.17,18 Then, remaining diagnosis and procedure codes are used to identify the 15 noncancer comorbidities in the Charlson Comorbidity Index.19 The algorithms used to identify these conditions reflect the Deyo et al20 adaptation of the Charlson Comorbidity Index and include several procedure codes from the Romano et al21 adaptation. A weight is assigned to each condition, and the weights are summed to obtain the index for each patient.
Socioeconomic information is not available for individual patients. Instead, we used median income, percentage of the population living in poverty, and percentage of those age 25 years or older with some college as indicators of the socioeconomic status of individual patients in the CLL cohort. (These are reported at the tract level in which the patient lives.) The size of the metropolitan statistical area was used to account for geographic variation.
We used Medicare claims to identify anemia, neutropenia, or thrombocytopenia before diagnosis and before infused therapy, using an approach similar to the one followed to construct the National Cancer Institute comorbidity index. Anemia was defined by the presence of a diagnosis code for anemia, a revenue center code or J-code for an erythropoiesis stimulating agent, or a revenue center code or Healthcare Common Procedure Coding System code for a red blood cell transfusion. Thrombocytopenia and neutropenia were similarly defined using the condition-specific codes, except that no transfusion claims were included for neutropenia. Hemolytic anemia was identified for patients with an anemia diagnosis by the presence of at least one International Classification of Diseases, 9th Revision, Clinical Modification code for hemolytic anemia.
For exploration of hematologic outcomes after the initiation of infused therapy, we limited the attribution of anemia, neutropenia, and thrombocytopenia to situations where a specific intervention (ie, transfusion and/or drug therapy, as appropriate) was used within 180 days of initiating therapy. This was done because some persons had diagnosis codes for these conditions before initiating therapy, making subsequent use of these codes less meaningful. All-cause hospitalizations were identified using Part A claims data. Identification of the subgroup of infection-related hospitalizations was based on the Clinical Classifications Software algorithm (Agency for Healthcare Research and Quality).22
Statistical analysis
Unadjusted Kaplan-Meier survival plots were used to explore overall survival in the entire cohort based on the initial treatment group defined in the first 60 days. Results for the subset who survived this 60-day definition period were virtually identical (data not shown). Predefined Cox proportional hazards regression models were used to explore factors associated with both time to infused therapy and time to death. In the survival models, we used time-dependent covariates in the first 60 days after initiating infused therapy to classify patients into treatment groups. We did this in part to minimize the introduction of immortal-time bias into the analyses.23 We also used a delayed-entry approach for patients in the survival model, using time since diagnosis as the time scale, to more precisely control for this variable.24 Other covariates of interest included age, gender, race, education, poverty, advanced stage, comorbidity index, year of diagnosis or infusion (depending on model), and metropolitan statistical area size as discussed under “Patients characteristics.”
The primary multivariate analysis of survival compared rituximab plus chemotherapy with chemotherapy alone. Because of the variety of potential chemotherapy agents that could be used, we grouped them together as “chemotherapy” to reflect the real-world use of infused therapy. Because there was probably heterogeneity in the effects of therapy, we also conducted a variety of exploratory survival analyses: restricting to patients 70 years of age or older, stratification by advanced stage, and restricting the primary comparison to patients using fludarabine (ie, fludarabine with or without cyclophosphamide).
We also conducted survival analyses with all 3 treatment groups, using propensity scores as a method of accounting for the underlying factors affecting the treatment selection for each patient (ie, selection bias).25-27 Because there were 3 treatment groups, we used multinomial logistic regression to calculate a propensity score for each person, defined as the predicted probability of receiving each given treatment. This regression model was constructed using the covariates identified from the survival model, with the addition of time from diagnosis to initial infused therapy and SEER region as covariates. The resulting score accounts for patient characteristics (eg, age, gender, and race), disease severity (advanced stage and comorbidity burden), and both geographic and temporal variation, permitting treatment comparisons among patients with similar likelihoods of receiving each treatment. The scores were incorporated into the proportional hazards survival model as an inverse probability of treatment weights. In this model, only the 2 rituximab treatment groups were used as covariates, with the chemotherapy alone group as the reference. In addition, we used the propensity score as a continuous covariate as well as a categorical covariate, with virtually identical results (results not shown). We also trimmed the lowest and highest 5% of the sample to test for robustness to extreme values for the propensity score, but the findings were unchanged (results not shown).28
Logistic regression was used to evaluate the risk of hematologic outcomes within 180 days of the first infused therapy using similar models to those described for the survival model.29
Results
Patient population
We identified 6433 patients who met the study eligibility criteria, who had an average of 40 months of follow-up. The median age at diagnosis was 77 years (mean, 77.6 years). At the time of diagnosis, 1675 (21%) were advanced stage (ie, previous diagnosis of anemia or thrombocytopenia). Hemolytic anemia was present in 3.8% of the cohort at diagnosis. Table 1 contains additional demographic information.
Initial infused therapy
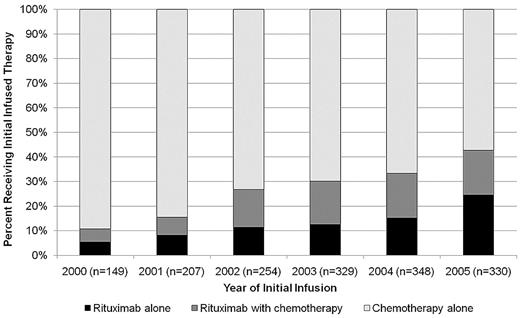
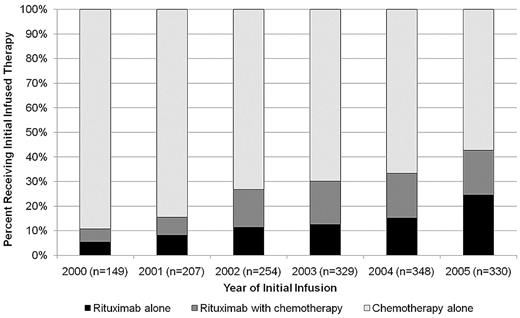
The median (50th percentile) time to first systemic therapy could not be estimated, but the 25th percentile was 676 days after diagnosis (95% confidence interval [CI], 613-751 days). As shown in Figure 1, the use of rituximab as a component of initial infused therapy increased from 10% for patients treated in 1999 to 43% for those treated in 2005.
Distribution of infused therapy by year of initiation. A total of 54 patients receiving therapy in 1999 were excluded because of small numbers. Year of infusion may be different from year of diagnosis.
Distribution of infused therapy by year of initiation. A total of 54 patients receiving therapy in 1999 were excluded because of small numbers. Year of infusion may be different from year of diagnosis.
Of the patients who received infused therapy (n = 2040), 1429 (70%) received chemotherapy alone, 319 (16%) received rituximab alone, and 292 (14%) received rituximab plus chemotherapy. Of the 292 patients receiving rituximab plus chemotherapy, 95 (32%) used a regimen including fludarabine. Of the remaining 197 patients, approximately half received a regimen containing cyclophosphamide. Of the 1429 patients receiving chemotherapy alone, 642 (45%) used a regimen including fludarabine. Of the remaining 787 patients, one-third received a regimen containing cyclophosphamide. Only 53 patients overall were identified as receiving both fludarabine and cyclophosphamide together (∼ 3% in each group, included in the fludarabine estimates).
On average, patients who received rituximab monotherapy and rituximab plus chemotherapy received 5.2 and 5.0 rituximab infusions, respectively, during the 180 days after initiating therapy (median, 4.0 and 4.5 days, respectively; interquartile range [IQR], 4-7 and 3-7, respectively). Patients receiving rituximab plus chemotherapy received 4.8 doses (IQR, 4-6) on average when they were advanced stage and 5.1 doses (IQR, 5-7) when they were not.
In multivariate-adjusted models of time to initial infused therapy, age more than or equal to 80 years at diagnosis, greater comorbidity burden, female gender, residing in areas with the highest poverty level, and “other” race/ethnicity were all associated with a significantly lower rate of initial infused therapy. In addition, patients with advanced disease at diagnosis were twice as likely to be treated with infused therapy (hazard ratio [HR] = 2.00; 95% CI, 1.82-2.20). See supplemental Data for additional details.
Survival analyses
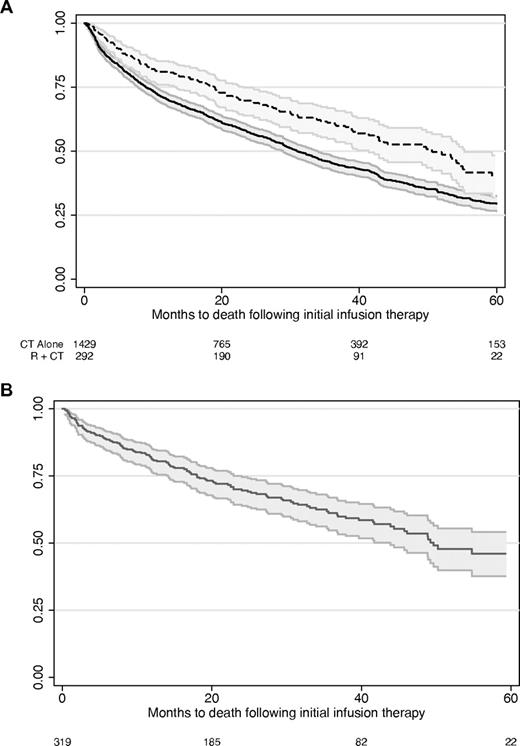
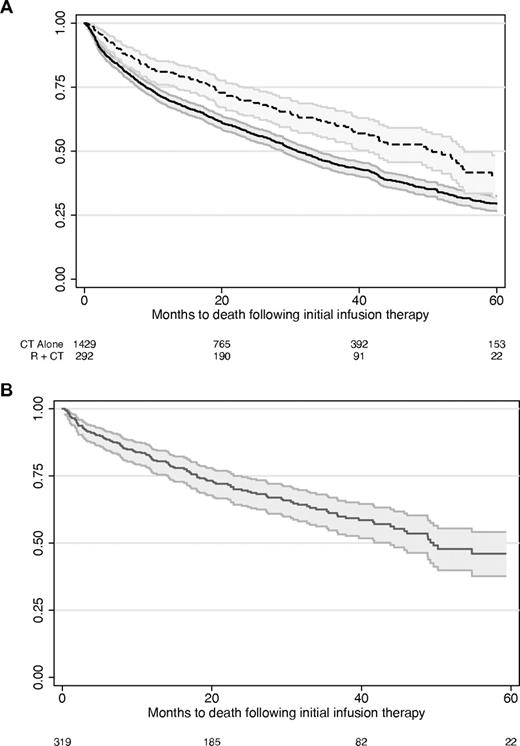
Median survival was 52 months (95% CI, 41-62 months) for rituximab plus chemotherapy and 34 months (95% CI, 31-38 months) for chemotherapy alone patients. Median survival was 53 months (95% CI, 44 months to undefined upper limit) for rituximab alone. The unadjusted Kaplan-Meier overall survival curves are shown in Figure 2.
Unadjusted overall survival in patients taking rituximab plus chemotherapy, chemotherapy alone, and rituximab monotherapy. (A) chemotherapy alone (CT alone) has the solid line (lower curve), and rituximab plus chemotherapy (R + CT) has the dashed line. (B) Rituximab monotherapy. Curves represent the survival estimate and the 95% confidence interval. Numbers at the bottom of the figures indicate the number of patients under observation at each time point.
Unadjusted overall survival in patients taking rituximab plus chemotherapy, chemotherapy alone, and rituximab monotherapy. (A) chemotherapy alone (CT alone) has the solid line (lower curve), and rituximab plus chemotherapy (R + CT) has the dashed line. (B) Rituximab monotherapy. Curves represent the survival estimate and the 95% confidence interval. Numbers at the bottom of the figures indicate the number of patients under observation at each time point.
Rituximab plus chemotherapy versus chemotherapy alone
In the primary multivariate-adjusted survival model (n = 1721), rituximab plus chemotherapy was associated with a 25% lower risk of death compared with chemotherapy alone (HR = 0.75; 95% CI, 0.62-0.91). Other factors associated with increased mortality risk include higher age, male gender, black race, advanced stage, and higher comorbidity burden (Table 2). Socioeconomic variables were not associated with mortality risk in these models. Fludarabine use (independent of other treatments) was added in an ad hoc model and was associated with a 14% reduction in mortality risk (HR = 0.86; 95% CI, 0.76-0.99).
Several additional models were used to explore factors affecting the main results. For patients 70 years of age or older, the results were similar to the overall model (HR = 0.71; 95% CI, 0.58-0.88). Rituximab plus chemotherapy was particularly effective in patients who were not advanced stage (HR = 0.57; 95% CI, 0.43-0.76). Patients with advanced-stage disease showed no statistically significant benefit from rituximab plus chemotherapy compared with chemotherapy alone (HR = 0.98; 95% CI, 0.75-1.29).
We also restricted the primary analysis to patients using fludarabine-containing regimens (n = 737). In this subpopulation, rituximab plus fludarabine-containing chemotherapy was associated with a 42% reduction in mortality compared with fludarabine-containing chemotherapy alone (HR = 0.58; 95% CI, 0.40-0.84).
Propensity score adjustment using the entire cohort
All treated patients were included in the primary propensity score survival model (n = 2040). Compared with patients receiving chemotherapy alone, those receiving rituximab plus chemotherapy (HR = 0.68; 95% CI, 0.57-0.82) and those receiving ritixumab alone (HR = 0.80; 95% CI, 0.68-0.93) showed significantly improved survival. In the subset of patients 70 years of age or older, the HRs for rituximab plus chemotherapy (HR = 0.70; 95% CI, 0.58-0.86) and rituximab alone (HR = 0.78; 95% CI, 0.66-0.92) were similar to the overall estimates.
Other outcomes
Hematologic interventions were relatively common during the 180 days after initiating infused therapy, particularly for anemia. The unadjusted rates are provided in Table 3 and are stratified by whether the condition was present or absent at any time before initiating infused therapy. The unadjusted risks of hospitalization and infection-related hospitalization are also included in Table 3. Multivariate-adjusted models are included in supplemental Data and summarized in the following paragraphs.
Using an adjusted model, the odds of receiving treatment for anemia were 55% higher in patients taking rituximab plus chemotherapy compared with those taking chemotherapy alone (odds ratio [OR] = 1.56; 95% CI, 1.17-2.04). Other risk factors for receiving anemia intervention included age more than 80 years and having had a diagnosis of anemia before therapy (OR = 2.80; 95% CI, 2.28-3.45). Patients in rural areas were 59% less likely to receive therapy for anemia (OR = 0.41; 95% CI, 0.22-0.76). Rituximab alone was associated with a lower anemia treatment risk compared with chemotherapy alone (OR = 0.36; 95% CI, 0.27-0.47).
Similar results were seen for thrombocytopenia interventions. Rituximab plus chemotherapy was associated with 63% higher odds of receiving intervention compared with chemotherapy alone (OR = 1.63; 95% CI, 1.02-2.59). Having a diagnosis of thrombocytopenia before first infused therapy was associated with an almost 4-fold increase in the odds (OR = 3.80; 95% CI, 2.48-5.82). Rituximab alone was associated with a lower thrombocytopenia treatment risk compared with chemotherapy alone (OR = 0.49; 95% CI, 0.25-0.94). Blacks were 78% less likely to receive treatment (OR = 0.22; 95% CI, 0.05-0.93).
In terms of risk of receiving neutropenia therapy, the use of rituximab plus chemotherapy was associated with a 2-fold higher risk compared with chemotherapy alone (OR = 1.98; 95% CI, 1.42-2.75). Having neutropenia before first infused therapy was associated with a much higher risk as well (OR = 4.15; 95% CI, 2.31-7.45), although this was very uncommon. Older ages and later years of initial treatment were both associated with a significantly lower risk of receiving therapy.
Discussion
In older patients with CLL, rituximab was part of the initial infused therapy for 30% of patients, approximately half of whom appeared to be receiving monotherapy. In addition, rituximab use increased over time, underscoring the importance of evaluating its real-world use in terms of both survival and potential adverse consequences of treatment. When rituximab plus chemotherapy was compared with chemotherapy alone (where chemotherapy was broadly defined), there was a 25% reduction in the overall risk of death for older patients using rituximab. Exploration of subgroups of patients showed that those with less advanced disease or those taking fludarabine as part of their chemotherapy regimen had larger risk reductions. In addition, age more than or equal to 70 years was not associated with differential effectiveness of rituximab in these analyses.
These results are similar to a previous observational study of R-FC that included a historical control group of FC patients.30,31 The median age in this previous study was 57 years, and only 41 patients (14%) were 70 years of age or older. Comparing the overall survival results for R-FC versus FC (HR = 0.48) in that study with the results from our fludarabine subgroup analysis (HR = 0.58) suggests that these results do extend to older patients. Similarly, patients in the CLL8 trial had a median age of 61 years, and only 10% of patients were age 70 or older. The comparison of R-FC with FC alone in this randomized, controlled trial showed a hazard ratio of 0.67 for overall survival, comparable with the 2 observational study estimates.5 Taken together, these findings help support the importance of therapy in improving CLL survival, as suggested by others.32-34
A related finding from the CLL8 trial was that patients with advanced disease had no survival benefit from the addition of rituximab, and this was consistent with our study. This is apparently not related to the number of rituximab doses, which were similar between the advanced and nonadvanced patients in both the CLL8 trial and our cohort. However, unlike the trial data, we could not identify dose reductions or consider other unfavorable prognostic factors that might be correlated with advanced stage and adjust our analyses accordingly. The CLL8 study showed that dose reductions and adverse prognostic factors were both more common in advanced-stage patients, which may explain these results in part.
The propensity score analyses supported our primary findings and showed that both rituximab plus chemotherapy and rituximab alone were associated with improved mortality compared with chemotherapy alone. It should be noted that the rituximab alone population was older, less likely to have advanced disease, and more likely to have hemolytic anemia. Compared with the other 2 groups, patients receiving rituximab alone had substantially fewer hematologic interventions, fewer hospitalizations, and excellent relative survival. These findings suggest that one should exercise caution in interpreting the propensity score-adjusted results. It still may not be appropriate to compare these patients directly with the population receiving chemotherapy (with or without rituximab) because of residual selection bias.25,26
However, the rituximab monotherapy survival results are important because they represent a substantial cohort in terms of size and follow-up for a therapeutic approach that has not been studied in great detail.35-37 One implication of these findings is that a trial of rituximab monotherapy may be a useful endeavor, particularly because it is already included in the National Comprehensive Cancer Network guidelines and is, as we have shown, commonly used. One hypothesis is that rituximab monotherapy may have been used in place of chlorambucil in some lower-risk patients or in combination with chlorambucil in some patients; however, we cannot make this determination with our data because chlorambucil is an oral drug that is not currently available in the SEER-Medicare data. This is another reason to interpret the rituximab monotherapy results carefully.
Hematologic interventions were required more often for patients taking rituximab plus chemotherapy compared with chemotherapy alone. In absolute terms, the differences were not large, particularly considering that these patients represent the complete spectrum of disease severity. In particular, patients whose physicians recognized these conditions before initiating infused therapy were much more likely to require intervention. Often, such high-risk patients are not included in clinical trials, so quantifying their risk of requiring intervention is important to making tradeoffs in the choice of appropriate therapy. (It is also important to note that ORs overestimate relative risks for common conditions, so the adjusted ORs should be interpreted in the context of the absolute risks in Table 3.) In addition, hospitalizations were not different between rituximab plus chemotherapy and chemotherapy alone. This suggests that, although hematologic outcomes may vary according to a variety of factors including treatment, they do not appear to place patients at elevated risk of hospitalization.
The selection of treatments in this population was affected by several factors that deserve mention. First, the rate of rituximab use increased substantially over time. This is not surprising given that rituximab became the standard of care for lymphoma during this period, but definitive trial data have only recently been published in CLL.38,39 In addition, the likelihood of treatment varied with poverty, race, and age, which is not unlike patterns seen in non-Hodgkin lymphoma and other areas of oncology.40,41 Although one might expect variation in practice patterns, which is common in areas where there is uncertainty about the relative benefits of competing treatments, race, age, and poverty-related imbalances in the diffusion of new technology raise concerns.42,43 Additional research into the factors associated with the uptake of rituximab in lymphoma may shed additional light on this issue.
As with any observational study, it is possible that the observed associations are the result of unobserved differences among patients (confounding). In particular, these data do not contain information on β2-microglobulin, lactate dehydrogenase, white cell count, or chromosome 17 abnormalities, all of which have been shown to be associated with survival.30 The concordance of our results and previously published results suggests that such omissions may not have had a substantive effect on the comparison between rituximab plus chemotherapy and chemotherapy alone. However, as discussed later in “Discussion,” there still may be potential selection bias issues, particularly for rituximab monotherapy.
Other limitations include our grouping of all chemotherapy regimens together rather than dividing them into specific regimens (eg, R-FC). Given the nature of the administrative data, it was not possible to identify specific regimens, although we were able to identify subsets of patients receiving commonly used agents (eg, fludarabine). The use of such a heterogeneous group would tend to bias the results toward no effect, something that was confirmed when we restricted our analyses to a more homogeneous group receiving fludarabine as part of its regimen. As already noted, oral medications without intravenous equivalents, such as chlorambucil, are not included in the data. As a result, some patients who relapsed after initial oral therapy might be included in our cohort.
The use of both propensity scores and traditional covariate adjustment in this analysis also deserves comment. The propensity scores are intended to account for the underlying factors that affect the selection of treatment for each patient and to summarize them in a single score. In theory, if this selection is properly accounted for, one can then compare patients with similar likelihoods of receiving a given treatment in much the same way as is done in clinical trials (ie, using the propensity score to balance the various treatment groups in place of randomization). For the most part, the use of this 2-part approach yields results comparable with those achieved using a single traditional multivariate model, although this is not always the case.26,27,44,45 When the results from our traditional analyses are compared with those using propensity scores, the findings are comparable for rituximab plus chemotherapy. With propensity score adjustment, the mortality risk reduction is slightly larger (32%) compared with the traditionally adjusted model (25%). Interestingly, the difference is larger and in a different direction for rituximab monotherapy: The propensity score analysis estimated a 20% mortality risk reduction, whereas the traditional, multivariate-adjusted model estimated a 40% risk reduction (supplemental Data). This highlights the importance of using multiple approaches to adjustment and suggests that additional studies in this population are needed to define this finding more precisely.
Another methodologic note is that the hematologic outcomes in our study are not the same as grade 3 or 4 adverse events reported in clinical trials. We relied on the use of interventions to define hematologic outcomes; in contrast, clinical trials use established and prespecified guidelines to characterize laboratory abnormalities and to determine whether they are drug-related. Furthermore, our population is much more heterogeneous than typical trial populations, making comparisons with trial-based adverse event rates more challenging. Finally, our hematologic outcomes reflect not only the underlying laboratory values, but also variations in practice patterns in using interventions; these 2 factors cannot be easily disentangled.
In conclusion, our findings suggest that initial treatment with rituximab was associated with improved survival in a heterogeneous group of more than 2000 older CLL patients, particularly in patients using fludarabine-based chemotherapy. Although rituximab was associated with an increase in the use of cytopenia interventions, it was not associated with an increase in overall hospitalizations. Further clinical studies may be warranted in an older patient population to confirm these findings, particularly those related to rituximab monotherapy. These analyses, although based on observational data and not clinical trials, suggest that the benefits of therapy in the older population are comparable with those demonstrated both in earlier observational studies and in more recent clinical trials.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Genentech Inc through a contract with Outcomes Insights Inc. This contract specifies that the authors are free to publish findings based on this research without restriction.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers For Medicare and Medicare Services (CMS); Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Authorship
Contribution: M.D.D. and R.I.G. acquired the data; M.D.D., R.I.G., and M.G. developed the initial analysis plan; K.K., S.S.-H., J.M., and C.R. provided comments on the analysis plan; M.G. did the programming; K.K. and J.M. provided additional clinical input during data analysis; M.D.D. wrote the initial version of the manuscript; M.G. created all tables and figures; and all authors contributed to the revisions of the manuscript.
Conflict-of-interest disclosure: M.D.D., R.I.G., and M.G. work for Outcomes Insights Inc in a research and consulting capacity. S.S.-H. and K.K. are consultants to Genentech Inc. C.R. is an employee of Genentech Inc. The remaining author declares no competing financial interests.
Correspondence: Mark D. Danese, 340 N Westlake Blvd, Suite 200, Westlake Village, CA 91362; e-mail: mark@outins.com.