Abstract
The clinical value of multiparameter flow cytometry (MFC) immunophenotyping in primary or light chain amyloidosis (AL) remains unknown. We studied 44 consecutive bone marrow samples from newly diagnosed patients with amyloidosis; 35 patients with AL and 9 with other forms of amyloidosis. Monoclonal plasma cells (PCs) were identifiable by MFC immunophenotyping in 34 of 35 (97%) patients with AL, whereas it was absent from all but 1 of the 9 (11%) patients with other forms of amyloidosis. Quantification of bone marrow plasma cells (BMPCs) by MFC immunophenotyping was a significant prognostic factor for overall survival (OS) (≤ 1% vs > 1% BMPC cutoff; 2-year OS rates of 90% vs 44%, P = .02). Moreover, detecting persistent normal PCs at diagnosis identifies a subgroup of patients with AL with prolonged OS (> 5% vs ≤ 5% normal PC within all BMPC cutoff, 2-year rates of 88% vs 37%, P = .01). MFC immunophenotyping could be clinically useful for the demonstration of PC clonality in AL and for the prognostication of patients with AL.
Introduction
Primary or light chain amyloidosis (AL) is the most frequent type of systemic amyloidosis and is characterized by the accumulation of monoclonal light chain fragments, leading to end-organ damage and short survival.1-4 Other forms of amyloidosis, such as localized, inherited, or secondary, are associated with different clinical courses and therapies.5,6 In contrast to these other forms of amyloidosis, in AL bone marrow (BM), plasma cells (PCs) are responsible for fibril deposition6 ; however, the bone marrow plasma cell (BMPC) infiltration is usually low,7 and clonality is not always apparent.8,9 Multiparameter flow cytometry (MFC) immunophenotyping has shown to be of clinical value in multiple myeloma (MM),10-13 but its use in other PC disorders such as AL remains largely unexplored.14-18 In this study, we investigate the clinical utility and prognostic value of MFC immunophenotyping in patients with AL.
Methods
Patients, material, and methods
We evaluated 44 consecutive BM samples from patients with newly diagnosed amyloidosis referred to our institution for immunophenotypic investigations. Samples were collected after informed consent was given, in accordance with Hospital Universitario Salamanca ethical committee guidelines and the Declaration of Helsinki. From the 44 cases, 35 had a confirmed diagnosis of AL based on the presence of an amyloid-related systemic syndrome, positive amyloid tissue staining with Congo red, and evidence of a monoclonal PC (M-PC) proliferative disorder at the cellular or serologic level. The remaining 9 cases were diagnosed with localized (n = 6), secondary (n = 2), or familial (n = 1) forms of amyloidosis. Table 1 shows the demographics and disease characteristics of the 35 patients with AL; 20 were treated with chemotherapy (melphalan-prednisone or melphalan-dexamethasone), whereas 10 received an autologous stem cell transplant; 5 patients were deemed too ill to tolerate any therapy.
Demographics and baseline characteristics of patients with light-chain amyloidosis (AL)
| Characteristic . | Value . |
|---|---|
| No. of patients | 35 |
| Male/female* | 24 (69)/11 (31) |
| Age, y† | 64 (38-90) |
| Clonality, λ* | 26 (74) |
| Number of organs involved* | |
| 1-2 | 23 (66) |
| 3-4 | 12 (34) |
| Kidney involvement* | 30 (86) |
| Heart involvement* | 18 (51) |
| Liver involvement* | 5 (14) |
| Peripheral nerve involvement* | 9 (26) |
| Serum albumin, g/dL† | 2.9 (1.0-5.7) |
| Serum creatinine, mg/dL† | 1.3 (0.5-7.0) |
| Serum alkaline phosphatase, IU/L† | 100 (49-459) |
| β2-Microglobulin, mg/L† | 3.6 (1.3-15.0) |
| Urine total protein, g/24 h† | 5.0 (0-45) |
| Serum M-component, g/dL† | 0.7 (0-2.9) |
| Urine M-component, g/24 h† | 0.2 (0-9.5) |
| Serum free light chain, mg/L† | 159 (22-513) |
| Serum cardiac troponin T, ng/mL† | 0.01 (0-0.3) |
| NT-ProBNP, pg/mL† | 1773 (27-14 365) |
| PCs by morphology† | 4.0 (0.1-24.0) |
| PCs by MFC immunophenotyping† | 1.4 (0.1-15.0) |
| Characteristic . | Value . |
|---|---|
| No. of patients | 35 |
| Male/female* | 24 (69)/11 (31) |
| Age, y† | 64 (38-90) |
| Clonality, λ* | 26 (74) |
| Number of organs involved* | |
| 1-2 | 23 (66) |
| 3-4 | 12 (34) |
| Kidney involvement* | 30 (86) |
| Heart involvement* | 18 (51) |
| Liver involvement* | 5 (14) |
| Peripheral nerve involvement* | 9 (26) |
| Serum albumin, g/dL† | 2.9 (1.0-5.7) |
| Serum creatinine, mg/dL† | 1.3 (0.5-7.0) |
| Serum alkaline phosphatase, IU/L† | 100 (49-459) |
| β2-Microglobulin, mg/L† | 3.6 (1.3-15.0) |
| Urine total protein, g/24 h† | 5.0 (0-45) |
| Serum M-component, g/dL† | 0.7 (0-2.9) |
| Urine M-component, g/24 h† | 0.2 (0-9.5) |
| Serum free light chain, mg/L† | 159 (22-513) |
| Serum cardiac troponin T, ng/mL† | 0.01 (0-0.3) |
| NT-ProBNP, pg/mL† | 1773 (27-14 365) |
| PCs by morphology† | 4.0 (0.1-24.0) |
| PCs by MFC immunophenotyping† | 1.4 (0.1-15.0) |
NT-ProBNP indicates N-terminal prohormone of brain natriuretic peptide.
Results expressed as number (percentage) of cases.
Results expressed as median (range).
Erythrocyte-lysed whole BM samples were stained by 4-color direct immunofluorescence, as described elsewhere.10-12,19 The monoclonal antibody combination (fluorescein isothiocyanate/phycoerythrin/peridinin chlorophyll protein-cyanin 5.5/allophycocyanin) CD38/CD56/CD19/CD45 allowed the identification, quantification, and discrimination between M-PCs and normal PCs (N-PCs) in more than 90% of patients with a sensitivity of 10−4.19,20 For the remaining patients, additional staining with cytoplasmatic immunoglobulin (Ig)κ/Igλ and surface CD38 plus CD19, CD45, or CD56 was performed. Cell cycle was analyzed as previously described13 in 10 of the 35 patients with AL. Cells were acquired in a FACSCalibur flow cytometer (BD Biosciences) using the CellQuest program (BD Biosciences), and information was recorded for ≥ 3 × 103 BMPCs/tube. Data were analyzed using the Paint-A-Gate-PRO 3.0 program (BD Biosciences).11,19
The χ2 and Mann-Whitney U tests were used to estimate the statistical significance of differences. Survival curves were plotted using the Kaplan-Meier method, and differences were assessed with the log-rank test. SPSS software Version 18.0 (SPSS) was used for all analyses.
Results and discussion
We detected phenotypically aberrant M-PCs by MFC in 34 of the 35 (97%) patients with AL. By contrast, M-PCs were only detectable in the BM of 1 of 9 (11%) cases with other forms of amyloidosis (P < .001). The presence of M-PCs in a patient with localized (duodenal) amyloidosis is intriguing, although this case may be representative of the nearly 3% of adults with monoclonal gammopathy of undetermined significance (MGUS), and thus exhibiting a clonal PC process.21 This suggests that the demonstration of PC clonality by MFC in patients with amyloid-positive tissue staining could be useful for the differential diagnosis of AL from other forms of amyloidosis. The sensitivity of MFC for the screening of AL in this study (97%) was similar to that achieved with serum and urine electrophoresis plus immunofixation (94%-96%),22,23 even when additional serum free light chain (sFLC) measurements are also performed (98%).22 Conversely, the sensitivity of sFLC without serum and urine electrophoresis plus immunofixation drops considerably, with around 12% of patients with AL displaying normal sFLC ratios at diagnosis.22,24 There are 3 possible explanations for the high sensitivity of MFC immunophenotyping for demonstrating PC clonality in AL: (1) MFC immunophenotyping is independent of the amount and type of secreted M-component, rate of kidney excretion, and tissue deposition; (2) MFC immunophenotyping is independent of the amount of polyclonal Ig produced; and (3) the higher sensitivity of MFC immunophenotyping compared with immunohistochemistry makes it less dependent on the clone size.25
Next, we wanted to examine further the immunophenotypic characteristics of M-PCs in patients with AL. Phenotypically aberrant M-PCs were detected mainly on the basis of simultaneous infra-expression of CD19 and CD45, with or without overexpression of CD56 (44% and 32% of cases, respectively). The remaining cases (24%) featured less commonly detected aberrant phenotypes (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Overall, these results are consistent with those of Matsuda et al15,16 from a shorter series (≤ 10 patients with AL). Interestingly, AL M-PCs showed an immunophenotypic profile that largely overlaps with MM M-PCs,10,11 although these entities have a clearly different clinical picture. We also explored the proliferative capacity of M-PCs in a subgroup of patients with AL. The median percentage of M-PCs in S-phase was 0.7% (range 0%-1.6%), which is lower than that observed in MM (1.6%-3.6%)12,13 and closer to that in smoldering MM (∼ 1%, our unpublished data).
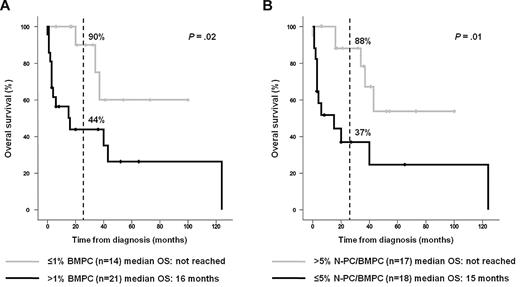
BMPC infiltration is typically low in AL,6,7 and probably because of this characteristic its percentage, assessed by morphologic and immunohistochemistry techniques, is not usually considered when predicting the overall survival (OS) of patients with AL.6 We have recently found that in MM, although MFC generally yields lower PC counts than conventional morphology, the former is an independent prognostic factor.12 In the present AL series, the median BMPC percentage measured by morphology was also higher (P < .001) than that obtained by MFC immunophenotyping (Table 1). However, whereas the number of PCs estimated by morphology did not influence OS (data not shown), patients with more than 1% BMPC measured by MFC immunophenotyping had a significantly shorter OS (2-year rates of 44% vs 90%, respectively; P = .02) than patients with 1% or less BMPC (Figure 1A). With respect to the potential prognostic value derived by the degree of clonality in AL, Kumar et al24 using the sFLC test have found that patients with normal baseline ratios have a longer OS than those with abnormal sFLC ratios.24 We have previously found that MFC immunophenotyping detection of more than 5% N-PC within the BMPC compartment (N-PC/BMPC) was useful for the differential diagnosis of MGUS and MM,10 and identification of patients with low-risk MGUS, smoldering MM, and symptomatic MM.11,19 In this series, 17 of 35 (49%) patients with AL had more than 5% N-PC/BMPC at diagnosis and, interestingly, these patients had a significantly longer OS (2-year rates of 88% vs 37%, respectively, P = .01) than patients with 5% or less N-PC/BMPC (Figure 1B). On excluding the 5 patients who did not receive any treatment, the prognostic influence of having more than 5% versus 5% or less N-PC/BMPC remained significant (P = .02); moreover, patients with more than 1% BMPC measured by MFC immunophenotyping also showed a trend for an adverse outcome (P = .07). Therefore, BMPC enumeration and particularly the detection of more than 5% N-PC/BMPC by MFC immunophenotyping could be of prognostic value in AL, similar to what was previously found in MGUS and MM.11,12,19 We have also compared the baseline characteristics of patients with more than 5% vs 5% or less N-PC/BMPC. No significant differences were found for conventional parameters, including the number and type of organs involved, except for the percentage of BMPCs quantified by MFC immunophenotyping (0.8% vs 2.8%, respectively, P < .001), whereas differences by morphology were borderline (4% vs 7%, P = .1). The median difference between involved and uninvolved free light chain (dFLC) was higher in cases with 5% or less N-PC/BMPC (627 mg/L) compared with patients with more than 5% N-PC/BMPC (150 mg/L), but differences failed to reach statistical significance (P = .2); regarding the frequency of patients in stage 2 and stage 3 AL defined by the N-terminal prohormone of brain natriuretic peptide and cardiac troponin T (Mayo Clinic staging system), no significant differences were observed on comparing patients with 5% or less versus more than 5% N-PC/BMPC (88% vs 60%; P = .7). The limited number of cases precluded a multivariate analysis to determine whether BMPC enumeration and detection of more than 5% N-PC/BMPC by MFC immunophenotyping are independent prognostic markers in AL.
Overall survival according to the MFC immunophenotypic evaluation of the BMPC compartment. The OS of patients with immunoglobulin light-chain amyloidosis (AL) grouped according to the percentage of BMPC detected at diagnosis by MFC immunophenotyping at cutoff value of > 1% BMPCs (A) and according to the percentage of normal PCs within all BMPCs (N-PC/BMPC) detected at diagnosis by MFC immunophenotyping at a cutoff value of > 5% N-PC/BMPC (B).
Overall survival according to the MFC immunophenotypic evaluation of the BMPC compartment. The OS of patients with immunoglobulin light-chain amyloidosis (AL) grouped according to the percentage of BMPC detected at diagnosis by MFC immunophenotyping at cutoff value of > 1% BMPCs (A) and according to the percentage of normal PCs within all BMPCs (N-PC/BMPC) detected at diagnosis by MFC immunophenotyping at a cutoff value of > 5% N-PC/BMPC (B).
In summary, our results raise the possibility that in patients with suspected amyloidosis, MFC immunophenotyping could be clinically useful for demonstrating PC clonality in AL versus other forms of amyloidosis and may also offer additional prognostication in AL; these results requiring validation in larger and prospective series.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Cooperative Research Thematic Cancer Network (RTIC) grants RD06/0020/0006 and G03/136, MM Jevitt, SL firm, Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria (FIS) grants PI060339, 02/0905, 01/0089/01-02, PS09/01897, and Consejería de Sanidad, Junta de Castilla y León, Valladolid, Spain grant 557/A/10.
Authorship
Contribution: B.P. and M.-B.V. designed the study protocol, collected and assembled data, analyzed and interpreted data, analyzed the flow cytometric data, performed statistical analysis, and wrote the manuscript; J.J.P., and M.-C.L.-B. analyzed and interpreted the flow cytometric data; R.G.-S, E.M.O., N.H., R.C., A.G.C., E.P., J.A., M.S., A.B., J.H., L.S., J.G., and M.-V.M. contributed with provision of study material or patients; and J.F.S.-M. conceived the idea, designed the study protocol, analyzed and interpreted data, and wrote the manuscript. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jesús F. San-Miguel, Hospital Universitario de Salamanca, Paseo de San Vicente 58-182, 37007 Salamanca, Spain; e-mail: sanmigiz@usal.es.
References
Author notes
B.P. and M.-B.V. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal