To the editor:
Hereditary erythrocytosis can be caused by mutations in genes involved in the hypoxia-inducible factor (HIF) pathway.1-3 For example, Chuvash polycythemia is caused by an R200W substitution in the von Hippel–Lindau protein (VHL).1 There is increasing evidence linking VHL-HIF dysregulation to altered vascular physiology, and a mouse model of Chuvash polycythemia develops pulmonary arterial hypertension (PAH).4-6 Recently, we reported an autosomal dominant erythrocytosis associated with an activating EPAS-1 (HIF-2A) mutation in which there was late-onset PAH in some family members.7 We now report a patient with severe erythropoietic dysregulation and PAH who is a compound heterozygote for novel VHL mutations.
A 2-month-old boy presented with increasing dyspnea and hypoxia requiring emergency ventilation and inotropic support. Echocardiography showed right ventricular dysfunction and hypertrophy. Severe PAH was confirmed by cardiac catheterization. Pulmonary artery systolic pressure was 91 mm Hg (approximately twice systemic values). Infusions of nitric oxide, prostacyclin, and sildenafil were required to allow discontinuation of ventilation. Treatment with vasodilators, diuretics, and bosentan was continued on eventual discharge from hospital.
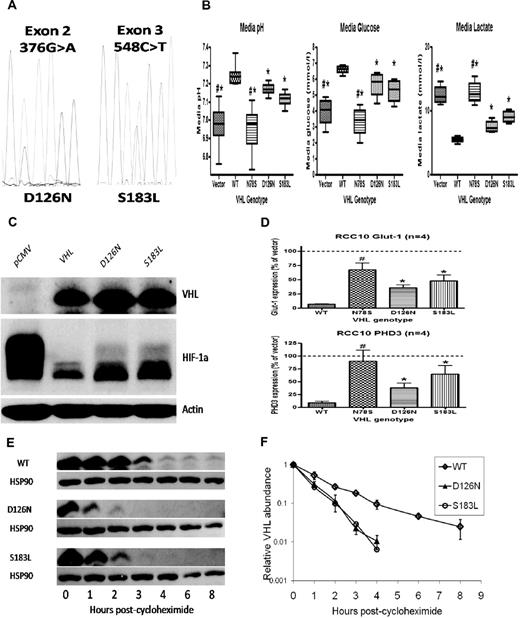
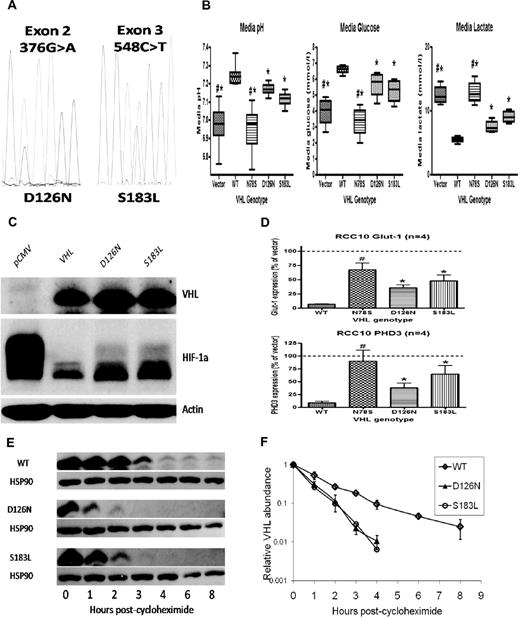
Consistently raised hemoglobin (Hb) concentrations (> 21 g/dL) prompted further investigation. Serum erythropoietin (EPO) concentration was grossly elevated at 4120 IU/L. Diagnostic imaging and selective venous sampling provided no evidence of an EPO-secreting lesion. We hypothesized that this unusual phenotype was explicable by congenital dysregulation of the HIF pathway. Gene sequencing revealed heterozygous mutations in exon 2 (376 G>A) and exon 3 (548 C>T) of VHL (Figure 1A), predicting the amino acid changes Asp126Asn (D126N) and Ser183Leu (S183L), respectively.
VHL mutations result in impaired VHL function and increased HIF activation. Mutant proteins are unstable in vitro. (A) Electropherogram showing G/A heterozygosity at base 376 (predicting a change from Aspartic Acid to Asparagine at residue 126) and C/T heterozygosity at base 548 (predicting a change from Serine to Leucine at residue 187). (B) RCC10 cell pools stably expressing either the D126N or S183L mutants show reductions in media pH, reduced levels of glucose and increased levels of lactate (consistent with up-regulated glycolytic metabolism) compared with pools expressing wild type (WT) protein. A nonfunctional VHL mutant (N78S) is presented as a comparison. (C) Western blotting shows increased levels of HIF-1α protein in lysates obtained from D126N and S183L RCC10 cell pools compared with WT RCC10 pools. (D) QPCR analysis of mutant RCC10 pools show increased expression of HIF-1α target genes compared with WT pools. (E) The rate of reduction in protein expression was measured after addition of cycloheximide to inhibit protein translation. This was increased in mutant 786-O cell pools. Quantification of VHL protein (after normalization to HSP90 loading control) is represented graphically in panel F.
VHL mutations result in impaired VHL function and increased HIF activation. Mutant proteins are unstable in vitro. (A) Electropherogram showing G/A heterozygosity at base 376 (predicting a change from Aspartic Acid to Asparagine at residue 126) and C/T heterozygosity at base 548 (predicting a change from Serine to Leucine at residue 187). (B) RCC10 cell pools stably expressing either the D126N or S183L mutants show reductions in media pH, reduced levels of glucose and increased levels of lactate (consistent with up-regulated glycolytic metabolism) compared with pools expressing wild type (WT) protein. A nonfunctional VHL mutant (N78S) is presented as a comparison. (C) Western blotting shows increased levels of HIF-1α protein in lysates obtained from D126N and S183L RCC10 cell pools compared with WT RCC10 pools. (D) QPCR analysis of mutant RCC10 pools show increased expression of HIF-1α target genes compared with WT pools. (E) The rate of reduction in protein expression was measured after addition of cycloheximide to inhibit protein translation. This was increased in mutant 786-O cell pools. Quantification of VHL protein (after normalization to HSP90 loading control) is represented graphically in panel F.
To examine the functional consequences of the mutations, VHL-null renal carcinoma cells were transfected to generate cell pools stably expressing wild type (WT) or mutant proteins (Figure 1C). Function was assessed by measurement of the pH of cell culture media. Impaired or absent VHL function results in more rapid acidification because of HIF-mediated enhancement of glycolysis and suppression of mitochondrial respiration.8,9 As expected, expression of WT VHL increased media pH while an inactivating VHL mutation (N78S) had no effect. In contrast, each of the D126N and S183L mutants exhibited an intermediate effect (Figure 1B). Pools expressing mutant proteins consumed more glucose and produced more lactate compared with WT, consistent with enhanced glycolytic metabolism (Figure 1B). To confirm that D126N and S183L mutations impair the ability of VHL to regulate HIF, we examined HIF-1α protein levels (Figure 1C) and the expression of HIF target genes PHD3 and GLUT-1 (Figure 1D), all of which were elevated in comparison to WT.
Thus, our patient has compound heterozygosity for novel mutations in VHL, which impair the ability to regulate HIF. Strikingly, EPO levels are greatly in excess of those observed in previous patients with inherited VHL-HIF dysfunction, suggesting that this patient has a more severe defect in HIF regulation. We observed that D126N and S183L were expressed at lower levels in transfected cells compared with WT. Because stably transfected cell pools exhibit a range of expression of the introduced protein, we examined this in multiple clonal sublines, with similar results. We hypothesized that this could reflect intrinsic differences in the stability of each protein. We measured the rate of reduction in protein level after inhibition of translation with cycloheximide. This was consistently increased in the D126N and S183L clones compared with clones expressing WT (Figure 1E-F). This instability is unlikely to contribute to impaired ability to regulate HIF in the complementation assays described above, in which the VHL proteins are expressed at much higher levels than in normal cells. However, we postulate that decreased stability of VHL in EPO-producing cells in vivo is the most likely explanation for the severity of the phenotype.
Now 8 years old, this patient undergoes regular phlebotomy to maintain an Hb of less than 16 g/dL. Pulmonary vascular measurements remain stable, with no evidence of ventricular dysfunction. He remains under surveillance for classic features of VHL disease, though has developed none to date.
Authorship
Acknowledgments: The authors are grateful to the patient and the patient's family and to Miss Kerry Baker for obtaining blood samples. Mammalian expression plasmids were kind gifts from Wilhelm Krek (Institute of Cell Biology, Zurich, Switzerland) and Alexander Hergovich (Friedrich Meischer Institute for Biomedical Research, Basel, Switzerland).
Contribution: J.B. and D.P.G. designed and performed experiments, analyzed and interpreted results, and wrote the paper; T.C., S.A. and J.d.B. performed experiments; D.M.G. and O.W. provided experimental advice and analyzed and interpreted results; and P.H.M. and P.A. supervised the project.
Conflict-of-interest disclosure: P.H.M. is a founder and scientific director of ReOx Ltd, which aims to develop PHD inhibitors. The remaining authors declare no competing financial interests.
Correspondence: Dr Jonathan Bond, 4th Floor, MRC Clinical Sciences Centre, Imperial College, Hammersmith Campus, Du Cane Rd, London W12 0NN, United Kingdom, e-mail: j.bond@imperial.ac.uk.