Abstract
This study evaluated shedding of the platelet collagen receptor, glycoprotein VI (GPVI) in human plasma. Collagen or other ligands induce metalloproteinase-mediated GPVI ectodomain shedding, generating approximately 55-kDa soluble GPVI (sGPVI) and approximately 10-kDa platelet-associated fragments. In the absence of GPVI ligands, coagulation of platelet-rich plasma from healthy persons induced GPVI shedding, independent of added tissue factor, but inhibitable by metalloproteinase inhibitor, GM6001. Factor Xa (FXa) common to intrinsic and tissue factor-mediated coagulation pathways was critical for sGPVI release because (1) shedding was strongly blocked by the FXa-selective inhibitor rivaroxaban but not FIIa (thrombin) inhibitors dabigatran or hirudin; (2) Russell viper venom that directly activates FX generated sGPVI, with complete inhibition by enoxaparin (inhibits FXa and FIIa) but not hirudin; (3) impaired GPVI shedding during coagulation of washed platelets resuspended in FX-depleted plasma was restored by adding purified FX; and (4) purified FXa induced GM6001-inhibitable GPVI shedding from washed platelets. In 29 patients with disseminated intravascular coagulation, mean plasma sGPVI was 53.9 ng/mL (95% confidence interval, 39.9-72.8 ng/mL) compared with 12.5 ng/mL (95% confidence interval, 9.0-17.3 ng/mL) in thrombocytopenic controls (n = 36, P < .0001), and 14.6 ng/mL (95% confidence interval, 7.9-27.1 ng/mL) in healthy subjects (n = 25, P = .002). In conclusion, coagulation-induced GPVI shedding via FXa down-regulates GPVI under procoagulant conditions. FXa inhibitors have an unexpected role in preventing GPVI down-regulation.
Introduction
In atherothrombotic disease, thrombus formation at arterial shear rates involves initial platelet adhesion and activation at sites of vascular injury/collagen exposure, and activation of coagulation that generates active thrombin and stabilizes the thrombus by fibrin.1 Bidirectional regulation of platelet function and coagulation is important in both the pathology of atherothrombotic diseases, such as heart attack or acute ischemic stroke, and in the clinical use of antiplatelet or anticoagulant drugs.2-5
The platelet collagen receptor, glycoprotein (GP)VI, is a promising target of antithrombotic therapy because it is not only critical for initiating platelet activation and thrombus formation at arterial shear rates but also promotes thrombus growth and stability because of effects on coagulation.6-9 In experimental models, depletion of platelet GPVI results in decreased responsiveness to collagen, impaired procoagulant activity and thrombin generation ex vivo and in vivo, and protection from tissue factor (TF)–induced thromboembolism.9-11 GPVI agonists also increase phosphatidylserine exposure and microparticle formation, whereas stimulation of platelets with a GPVI ligand (convulxin) and thrombin increase active factor X (FXa) and thrombin, regulated by a subpopulation of platelets with increased binding of coagulation factors (FV, FVIII, FIX, and FX).12-15
Collagen or other ligands also induce ectodomain shedding of GPVI, mediated by one or more metalloproteinases, including ADAM10.16-21 GPVI shedding and release of a soluble GPVI ectodomain fragment (sGPVI) may be a sensitive readout of receptor involvement because all GPVI is intact on the surface of normal circulating platelets, unless shedding is induced.21,22 In this study, we show that activation of coagulation can down-regulate GPVI expression at the platelet surface by inducing metalloproteinase-mediated shedding by a mechanism involving activation of FXa.
Methods
Reagents and antibodies
Purified human FX and FXa were obtained from Banksia Scientific. FX-deficient plasma was from Helena Laboratories. Reagents for measuring thrombin generation, Ca2+-containing Fluo-buffer, thrombin substrate, TF reagent, and thrombin calibrator (Thrombinoscope-BV) were purchased from Pathtech and used according to the manufacturer's instructions. Russell viper venom (RVV) was from Diagnostica-Stago. The ADAM10 inhibitor, GI254023, was the generous gift of Dr Andreas Ludwig (RWTH Aachen University, Germany). SCH79797 (protease-activated receptor-1 [PAR1] antagonist) was from Tocris-Cookson. PP2 (Src inhibitor) and N,N-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-[2-[(acetyloxy)methoxy]-2-oxoethyl]]-bis[(acetyloxy)methyl]ester (BAPTA-AM; intracellular Ca2+ chelator) were from Calbiochem. Oregon Green (OG)–fibrinogen was from Invitrogen. Fluorescein isothiocyanate-labeled anti–rabbit IgG was from Chemicon. Affinity-purified mouse anti-GPVI (1G5) and anti-GPIbα (AK2) monoclonal antibodies, rabbit polyclonal anti-GPVI cytoplasmic tail, anti-GPV cytoplasmic tail, and rabbit anti–P-selectin IgG were previously described.21,23,24
Coagulation inhibitors
Dabigatran, a selective thrombin inhibitor (Boehringer-Ingelheim) was used at 1.25μM final concentration (equivalent to 775 ng/mL), a dose known to block TF-induced thrombin generation in human platelet-poor plasma in vitro,25 and consistent with plasma concentrations (100-1000 ng/mL) reported to prolong or flatten thrombin clotting time, activated clotting time, and ecarin clotting time ex vivo in healthy controls, orthopedic surgery or cardiovascular patients receiving oral dabigatran.26-30 Rivaroxaban, a direct FXa inhibitor (Bayer), was used at 1 μg/mL (equivalent to ∼ 2μM), compared with doses known to prolong the prothrombin time and activated partial thromboplastin time (APTT; 0.23 and 0.69μM, respectively) in vitro,28 and completely block TF-induced thrombin generation in platelet-rich plasma (PRP) from healthy volunteers in vitro and ex vivo (0.1-1μM).31 Enoxaparin (Sanofi-Aventis) was used at 0.2 U/mL; and hirudin, a thrombin inhibitor (Sigma-Aldrich) was used at 5 to 20 U/mL, doses used previously to block thrombin generation or prolong activated clotting time and APPT.32-34
Patients and controls
Collection of blood was carried out using procedures approved by the Human Ethics Committee of the Alfred Hospital, Melbourne, Australia. Citrated plasma samples were collected from 25 randomly selected healthy donors, 33 patients with thrombocytopenia and elevated D-dimer, suspected to have disseminated intravascular coagulation (DIC), and 36 consecutive outpatients with chemotherapy- and/or cancer-related thrombocytopenia. Demographic and clinical data, standard laboratory tests, including platelet count, and coagulation-related assays (APTT, international normalized ratio, D-dimer, and fibrinogen) were collected for all patients. Diagnosis of DIC was confirmed in 29 patients, whereas chemotherapy- and/or cancer-related thrombocytopenia with no evidence of DIC served as a control group (Table 1).
Preparation of platelets and plasma
Citrated PRP was obtained by centrifuging blood collected in trisodium citrate (0.32% weight/volume final) at 100g for 20 minutes at room temperature. Platelet-poor plasma was obtained by centrifuging PRP at 1000g for 10 minutes. Washed platelets were isolated and resuspended in Tyrode buffer (0.36mM NaH2PO4, 5.5mM glucose, 138mM NaCl, 12mM NaHCO3, 1.8mM CaCl2, 0.49mM MgCl2, 2.6mM KCl, pH 7.4) as previously described.21
Thrombin generation and shedding of platelet GPVI in human plasma
Shedding of GPVI from platelets in plasma was measured by inducing coagulation in citrated PRP by recalcification in the absence or presence of added TF, and measuring either thrombin generation or sGPVI levels in parallel samples. Thrombin generation was measured at 20-second intervals over 60 minutes at 37°C using the calibrated automated thrombin generation assay. Generation of thrombin in a test sample is quantitated by comparing thrombin activity with a reference sample containing a known amount of mutant thrombin added to nonclotting plasma (“calibrator” thrombin cleaves the substrate but does not participate in coagulation and is not inhibited by antithrombin). Assays were performed with or without addition of known amounts of TF (0.5pM) plus phospholipids. Triplicate samples in 96-well plates consisted per well of 100 μL sample (80 μL PRP plus 20 μL 0.01M Tris-HCl, 0.15M NaCl, pH 7.4, TS buffer) plus 20 μL Fluo-buffer containing thrombin substrate (40 μL plus 1.6 mL Fluo-buffer). Parallel samples contained thrombin calibrator (20 μL/well in place of TS buffer). Instead of PRP, some assays used FX-deficient plasma with or without purified FX (10 μg/mL) reconstituted with 20 μL washed platelets (109/mL). To test the effect of inhibitors, some assays included (final concentrations) rivaroxaban (1 μg/mL), enoxaparin (0.2 U/mL), dabigatran (1.25μM), or hirudin (5 or 20 U/mL). In some assays, 1 U/mL thrombin or RVV (1:1 molar ratio with FX) was added to PRP. Shedding of platelet GPVI in clotting PRP was measured under the same conditions as thrombin generation assays. At different times, samples were made 10mM in ethylenediaminetetraacetic acid, centrifuged at 1000g for 10 minutes, and sGPVI was measured in the supernatant.
Measuring sGPVI
sGPVI in plasma or serum obtained from clotting samples or sGPVI in patient plasma was assessed in triplicate by enzyme-linked immunosorbent assay (ELISA).22 sGPVI was calculated as the mean ± SD compared with a standard curve using recombinant GPVI ectodomain in GPVI-depleted plasma.22 sGPVI measurements were performed blind to the identity of DIC or thrombocytopenia patients.
FXa-induced GPVI shedding
To examine the ability of FXa to induce shedding of GPVI, washed platelets (100 μL) at 5 × 108/mL were treated with 10 μg/mL human FX with or without RVV (1:1 molar ratio), or with constitutively active FXa, and then mixed with 1:1 nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Washed platelets were also treated with either 0.1 μg/mL convulxin (CVX) or 2mM N-ethylmaleimide (NEM), a metalloproteinase-activating reagent, to induce GPVI shedding. Samples were immunoblotted with anti-GPVI cytoplasmic tail IgG as described.21,23,35 To assess a requirement for intracellular signaling, washed platelets were treated for 1 hour with FXa and 10μM Src inhibitor PP2, 20μM BAPTA-AM, 1 μg/mL rivaroxaban, or 10μM PAR1 inhibitor SCH79797. Platelets treated with 10μM phorbol myristate acetate were included as a positive control. Samples were made 10mM ethylenediaminetetraacetic acid and centrifuged to pellet platelets. sGPVI in the supernatant was assessed by ELISA. Platelets were resuspended in Tyrode buffer containing 0.5% weight/volume bovine serum albumin. Platelets were mixed with 2 μg/mL anti–P-selectin IgG and fluorescein isothiocyanate–labeled anti–rabbit IgG (to assess surface P-selectin), or 2 μg/mL OG-fibrinogen (to assess active αIIbβ3) for 30 minutes and analyzed by FACSCalibur (BD Biosciences) using a platelet gate defined by forward/side scatter and positive staining with anti-GPIbα antibody (AK2).
Statistical analysis
sGPVI levels were assessed for normality and found to be well approximated by a normal distribution after log transformation. A comparison between the groups (DIC patients, thrombocytopenic controls, and healthy controls) was performed using χ2 test or Fisher exact test for categorical variables and Kruskal-Wallis or Mann-Whitney U test as appropriate for nonparametric continuous variables. Post-hoc comparisons were done using Dunn test. Correlation of sGPVI with coagulation markers was assessed using Spearman rank correlation. Multivariate analysis was performed to assess the effect of group on sGPVI levels accounting for age and platelet count using the PROC GLM procedure in SAS Version 9.2 (SAS Institute). For comparing DIC patients with thrombocytopenic controls, coagulation markers (fibrinogen and APTT) and underlying cause were also considered as confounding factors. The results from log-transformed data were reported as geometric means with 95% confidence intervals. Demographic data were summarized and reported as mean ± SD or proportions. A 2-sided P value of .05 was considered statistically significant.
Results
Coagulation induces metalloproteinase-mediated release of sGPVI
We previously showed that blood collected in a silica-coated clot activator vacutainer to generate serum resulted in significantly higher sGPVI levels (123.45 ± 4.4 ng/mL) compared with plasma from the same donor collected in anticoagulant tubes (29.05 ± 2.9 ng/mL).22 The increased sGPVI was not the result of any confounding effect of the clotting reaction on the ELISA measurement because serum sGPVI could be completely immunodepleted by an anti-GPVI antibody (1G5). Increased plasma sGPVI was also not the result of platelet-derived microparticles because sGPVI levels were unaffected by centrifugation at 100 000g. This suggested that coagulation could induce ectodomain shedding of platelet GPVI.
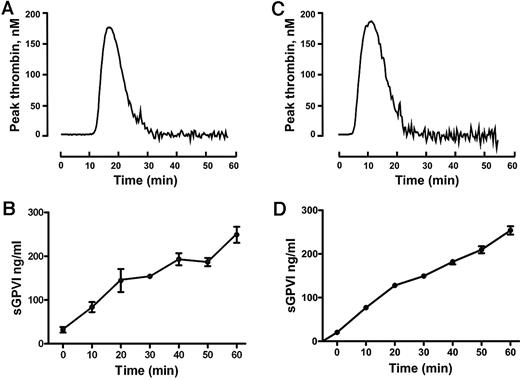
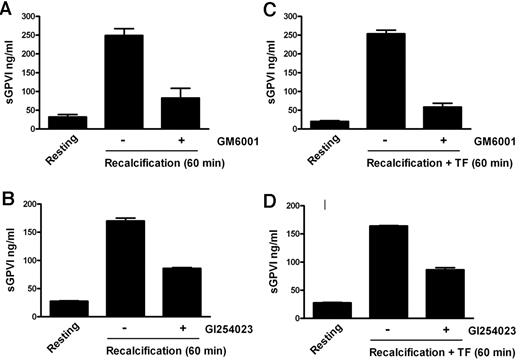
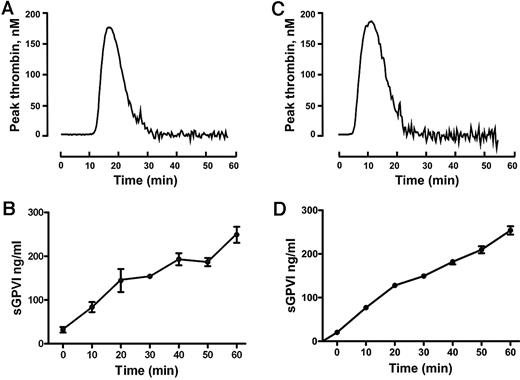
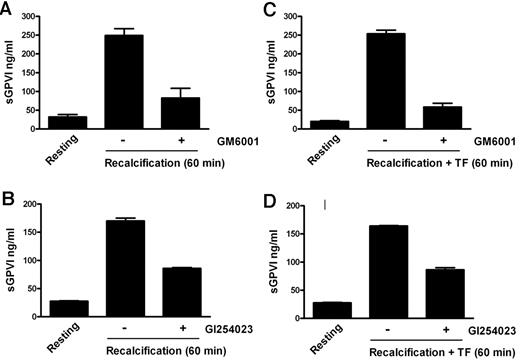
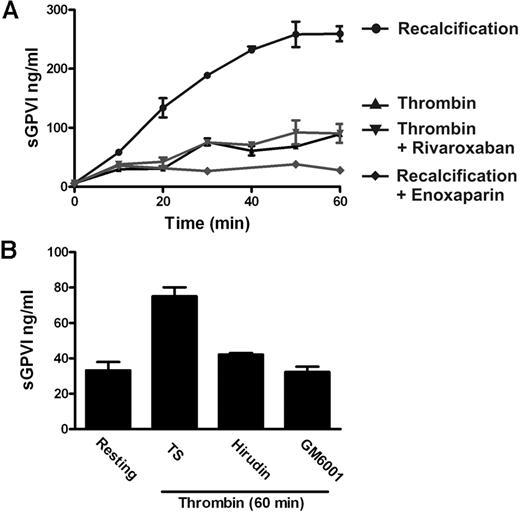
To confirm the ability of coagulation to induce GPVI shedding and investigate the mechanism for this process, we induced coagulation in citrated PRP by recalcification in the absence (intrinsic pathway) or presence of added 0.5pM TF plus phospholipid (extrinsic pathway). These samples, analyzed in parallel for thrombin generation or sGPVI, showed a time-dependent increase in sGPVI levels. There was an approximately 7- to 10-fold increase of sGPVI after 60 minutes, after the initiation of thrombin generation, without a requirement for added TF (Figure 1). Coagulation-induced GPVI shedding by recalcification with or without added TF was strongly inhibited by a metalloproteinase inhibitor, GM6001 (100μM), and partially inhibited by 2μM GI254023, a selective ADAM10 inhibitor (Figure 2), suggesting that sGPVI release was metalloproteinase-dependent and that ADAM10 plays an important role.
Release of sGPVI from platelets induced by coagulation in human plasma. Coagulation in citrated PRP was induced by recalcification at 37°C in the absence (A-B) or presence (C-D) of added TF plus phospholipids. Thrombin generation was measured using the calibrated automated thrombin assay (A,C). sGPVI was measured by ELISA, after addition of 10mM ethylenediaminetetraacetic acid and centrifugation for removal of platelets or the platelet-rich fibrin clot to generate serum (B,D). Data are representative of 3 independent experiments using PRP from 3 separate donors.
Release of sGPVI from platelets induced by coagulation in human plasma. Coagulation in citrated PRP was induced by recalcification at 37°C in the absence (A-B) or presence (C-D) of added TF plus phospholipids. Thrombin generation was measured using the calibrated automated thrombin assay (A,C). sGPVI was measured by ELISA, after addition of 10mM ethylenediaminetetraacetic acid and centrifugation for removal of platelets or the platelet-rich fibrin clot to generate serum (B,D). Data are representative of 3 independent experiments using PRP from 3 separate donors.
Coagulation-induced GPVI shedding is metalloproteinase-dependent. sGPVI measured in recalcified citrated PRP in the absence (A-B) or presence of added TF (C-D) obtained from samples either resting or preincubated with a metalloproteinase inhibitor 100μM GM6001 (A,C), or with the ADAM10 inhibitor 2μM GI254023 (B,D). Conditions were as described in Figure 1 legend.
Coagulation-induced GPVI shedding is metalloproteinase-dependent. sGPVI measured in recalcified citrated PRP in the absence (A-B) or presence of added TF (C-D) obtained from samples either resting or preincubated with a metalloproteinase inhibitor 100μM GM6001 (A,C), or with the ADAM10 inhibitor 2μM GI254023 (B,D). Conditions were as described in Figure 1 legend.
Thrombin is a relatively poor inducer of GPVI shedding
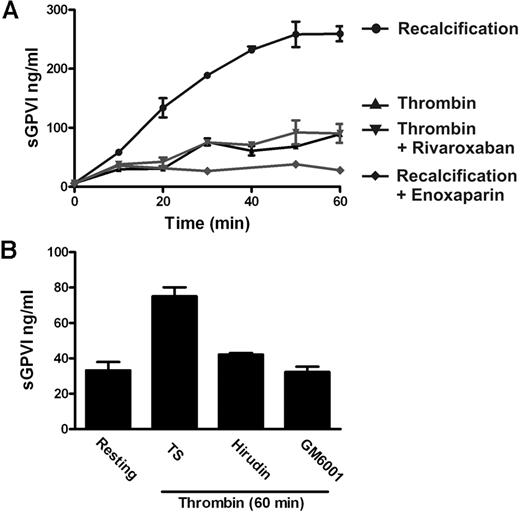
The findings that coagulation-induced GPVI shedding did not require added TF and that shedding commenced on a time scale after the initiation of thrombin generation indicated that factors, such as FX and thrombin, common to both intrinsic and extrinsic pathways, might be responsible for inducing GPVI shedding. In this regard, both published and present results suggest that thrombin is a relatively poor inducer of GPVI shedding. First, we originally showed that treating washed platelets with active thrombin did not induce detectable GPVI shedding under conditions where GPVI ligands induced substantial shedding.16 Thrombin receptor agonist peptide that, like thrombin, activates platelets via the protease-activated receptor PAR1 also failed to induce GPVI shedding under these conditions.36 Second, active thrombin added to citrated PRP induced far less sGPVI generation compared with shedding induced by activation of the whole coagulation cascade (Figure 3A). In this case, thrombin-induced GPVI shedding is not inhibited by the FXa inhibitor rivaroxaban, suggesting this shedding is not the result of any feedback amplification of coagulation leading to generation of FXa (Figure 3A). However, thrombin-induced GPVI shedding is inhibitable by the metalloproteinase inhibitor GM6001 and by the thrombin inhibitor hirudin (Figure 3B).
Active thrombin is a relatively poor inducer of GPVI shedding. (A) sGPVI measured in either recalcified citrated PRP in the absence or presence of 0.2 U/mL enoxaparin that inhibits FXa and thrombin, or citrated PRP treated with 1 U/mL thrombin in the absence or presence of 1 μg/mL rivaroxaban that selectively inhibits FXa. (B) sGPVI was measured in citrated PRP, either untreated or treated with thrombin in the absence or presence of 5 U/mL hirudin or 100μM GM6001.
Active thrombin is a relatively poor inducer of GPVI shedding. (A) sGPVI measured in either recalcified citrated PRP in the absence or presence of 0.2 U/mL enoxaparin that inhibits FXa and thrombin, or citrated PRP treated with 1 U/mL thrombin in the absence or presence of 1 μg/mL rivaroxaban that selectively inhibits FXa. (B) sGPVI was measured in citrated PRP, either untreated or treated with thrombin in the absence or presence of 5 U/mL hirudin or 100μM GM6001.
FXa is critical in coagulation-induced GPVI shedding
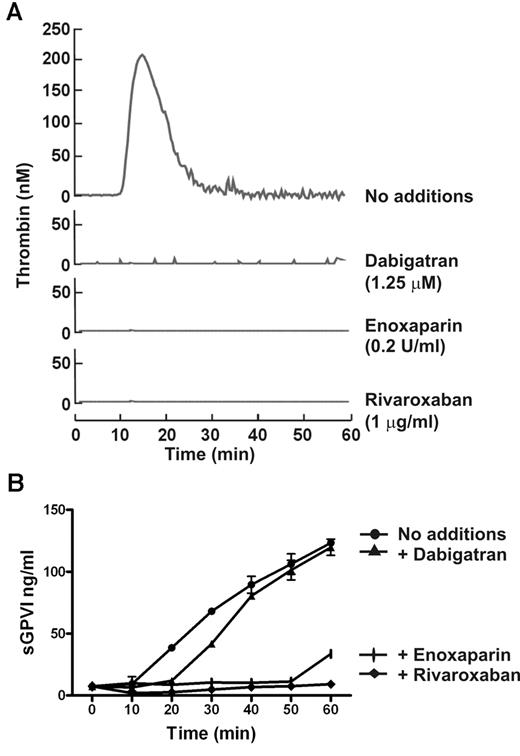
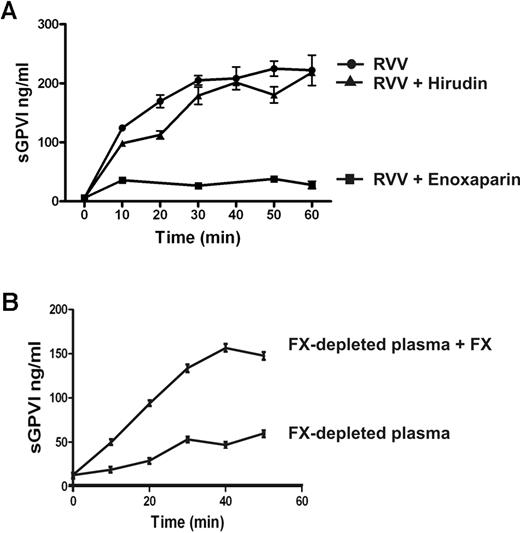
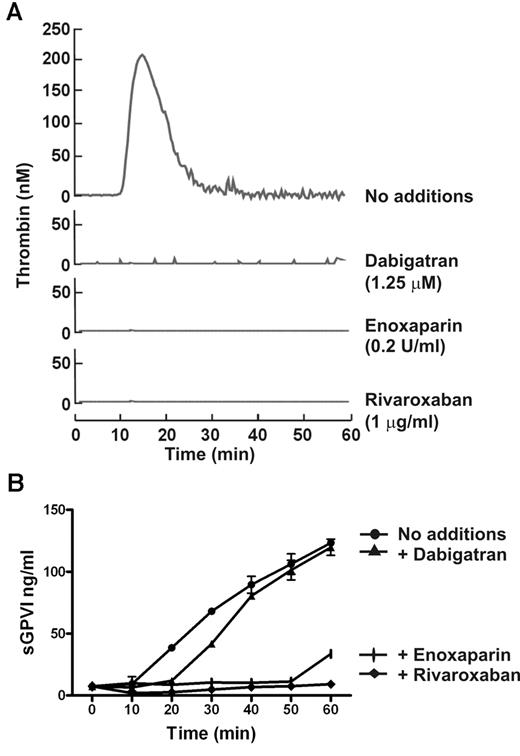
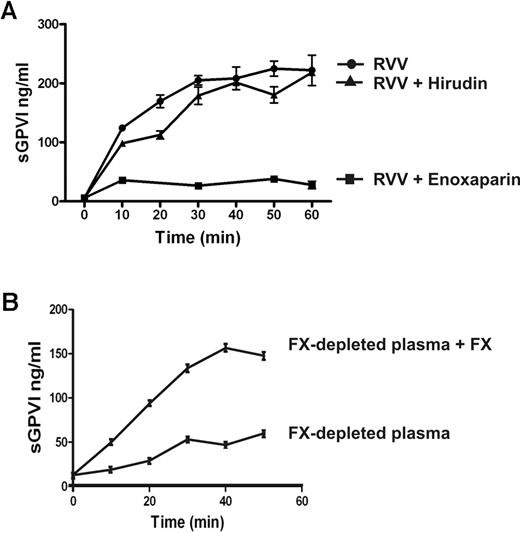
FX is activated to FXa immediately upstream of thrombin within the common coagulation pathway. To determine the possible role of FXa in sGPVI generation, coagulation in PRP was induced by recalcification in the presence of enoxaparin (0.2 U/mL) that blocks both FXa and thrombin (with a preference for inhibition of FXa over thrombin by an ∼ 4:1 ratio), dabigatran (1.25μM), a selective thrombin inhibitor,25,30,37 or rivaroxaban (1 μg/mL), a selective FXa inhibitor.38 sGPVI release was completely blocked by both rivaroxaban and enoxaparin (Figure 4) but only marginally by dabigatran or hirudin (Figure 4; and data not shown), suggesting that FXa is critical in GPVI shedding. Furthermore, direct activation of FX in PRP using RVV that directly generates FXa induced a comparable degree of sGPVI generation. RVV-induced shedding was completely blocked by enoxaparin that inhibits FXa and thrombin but was only weakly affected by the thrombin inhibitor hirudin (Figure 5A). Direct activation of FX by RVV in the presence of hirudin would rule out a possible contribution to GPVI shedding of upstream coagulation factors activated by thrombin feedback pathways. To confirm a role for FXa in GPVI shedding, resuspending washed platelets in FX-deficient plasma resulted in significantly impaired coagulation-induced sGPVI generation (Figure 5B). Adding back purified FX to FX-deficient plasma restored coagulation-induced sGPVI generation (Figure 5B). Together, these studies provide evidence that FXa is critical for coagulation-induced GPVI shedding in plasma, whereas thrombin may have a minor role.
FXa inhibitor blocks coagulation-induced GPVI shedding. Coagulation in citrated PRP induced by recalcification in the absence or presence of 0.2 U/mL enoxaparin (FXa and thrombin inhibitor), 1.25μM dabigatran (selective thrombin inhibitor), or 1 μg/mL rivaroxaban (selective FXa inhibitor). Thrombin generation assessed by the calibrated automated thrombin assay (A) was completely blocked by all inhibitors, whereas coagulation-induced sGPVI generation measured by ELISA (B) was blocked completely by rivaroxaban and enoxaparin, but only marginally attenuated by thrombin inhibitor, dabigatran.
FXa inhibitor blocks coagulation-induced GPVI shedding. Coagulation in citrated PRP induced by recalcification in the absence or presence of 0.2 U/mL enoxaparin (FXa and thrombin inhibitor), 1.25μM dabigatran (selective thrombin inhibitor), or 1 μg/mL rivaroxaban (selective FXa inhibitor). Thrombin generation assessed by the calibrated automated thrombin assay (A) was completely blocked by all inhibitors, whereas coagulation-induced sGPVI generation measured by ELISA (B) was blocked completely by rivaroxaban and enoxaparin, but only marginally attenuated by thrombin inhibitor, dabigatran.
Coagulation-induced GPVI shedding is mediated by FXa. (A) Coagulation induced by RVV (1:1 molar ratio with FX) in citrated PRP in the absence or presence of 0.2 U/mL enoxaparin or 20 U/mL hirudin. (B) Washed platelets (109/mL) resuspended in FX-deficient plasma with or without added purified FX (10 μg/mL, final concentration). Coagulation was induced by recalcification, and sGPVI was measured as described in Figure 1 legend.
Coagulation-induced GPVI shedding is mediated by FXa. (A) Coagulation induced by RVV (1:1 molar ratio with FX) in citrated PRP in the absence or presence of 0.2 U/mL enoxaparin or 20 U/mL hirudin. (B) Washed platelets (109/mL) resuspended in FX-deficient plasma with or without added purified FX (10 μg/mL, final concentration). Coagulation was induced by recalcification, and sGPVI was measured as described in Figure 1 legend.
FXa induces GPVI shedding in washed platelets
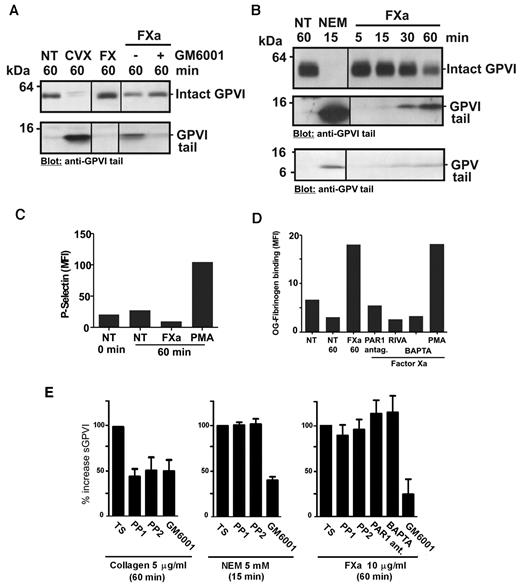
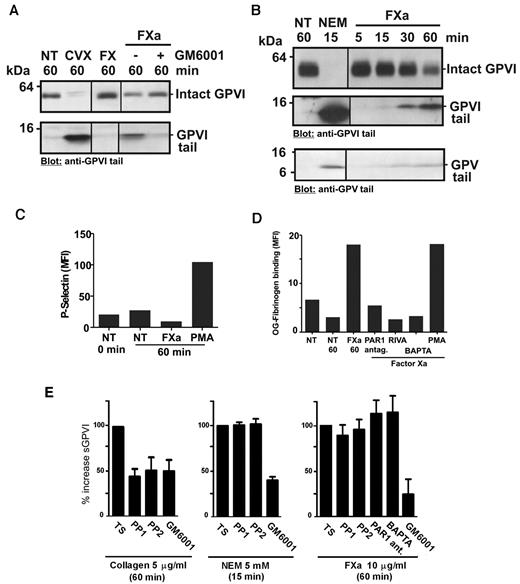
We previously demonstrated that an anti-GPVI tail IgG detects both intact GPVI (∼ 62 kDa) on untreated platelets and an approximately 10-kDa remnant fragment of GPVI on platelets after treatment with GPVI ligands, collagen or CVX, or thiol-modifying reagent, NEM, which directly activates metalloproteinases.16,36 Figure 6A and B show immunoblots of platelet lysates probed with anti-GPVI cytoplasmic tail IgG. FX plus activator RVV or purified FXa, but not FX alone, induced GPVI shedding from washed human platelets, with decreased intact GPVI and appearance of an ∼ 10-kDa fragment of GPVI. Treatment of platelets with FXa produced a time-dependent GPVI remnant fragment (Figure 6B) of the same molecular weight as fragments found in lysates from CVX- or NEM-treated platelets, and FXa-induced shedding was blocked by preincubation with GM6001, consistent with a metalloproteinase-dependent event (Figure 6A). Control experiments showed that GM6001 does not directly inhibit FXa activity because thrombin generation in recalcified PRP was not blocked by GM6001 at the same concentration (data not shown). Shedding was unlikely to be the result of a direct effect of FXa on GPVI because there was minor but detectable shedding of GPV from platelets when lysates were probed with anti-GPV cytoplasmic tail IgG (Figure 6B). However, there was no detectable loss of platelet surface GPIbα under the same conditions, when washed platelets treated for 60 minutes with FXa were compared with untreated platelets analyzed by flow cytometry with phycoerythrin-conjugated anti-GPIbα antibody, AK2 (Geomean values of 305 and 302, respectively). The effects of FXa treatment of washed platelets on other platelet activation events, including up-regulation of surface expression of P-selectin (Figure 6C) and αIIbβ3 activation assessed by OG-fibrinogen binding (Figure 6D), were evaluated by flow cytometry. Under these conditions where FXa treatment resulted in shedding of GPVI (Figure 6E), platelet P-selectin levels were not increased (Figure 6C). Activation of αIIbβ3 was detected in FXa-treated washed platelets (Figure 6D), consistent with other studies demonstrating cell activation via FXa activation of PAR1.39 Although this activation was specific for FXa (blocked by rivaroxaban) and blocked by pretreatment with either a PAR1 antagonist SCH79797 or the intracellular Ca2+-chelator BAPTA-AM, neither SCH79797, nor BAPTA-AM, nor Src kinase inhibitors, PP1 or PP2, affected FXa-induced GPVI shedding (Figure 6E). As expected, PP1/PP2 inhibited activation-dependent collagen-induced shedding, but not activation-independent shedding induced by NEM (Figure 6E). Taken together, these data indicate that FXa-induced shedding of GPVI does not require platelet intracellular signaling or activation of PAR1. The results also imply that phosphatidylserine exposure and procoagulant complexes involving FXa are not critical for GPVI shedding.
FXa induces GPVI shedding in washed platelets. Washed platelets resuspended at 5 × 108/mL in Tyrode buffer had no treatment (NT) or were mixed with (A) 0.1 μg/mL CVX, 10 μg/mL purified FX or FXa (10 μg/mL purified FX plus a 1:1 molar ratio of RVV to generate FXa) or (B) 5mM NEM or 10 μg/mL purified FXa. GM6001 (100μM) was added to platelets before addition of other factors where indicated. Platelet lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-GPVI cytoplasmic tail IgG or anti-GPV cytoplasmic tail IgG (B bottom panel), and visualized using chemiluminescence. A vertical line indicates a repositioned gel. Washed platelets (107/mL in Tyrode buffer) were pretreated with 10μM PP1 or PP2, 20μM BAPTA-AM, 1 μg/mL rivaroxaban (RIVA), or 10μM PAR1 inhibitor SCH79797 and then mixed with 10 μg/mL purified FXa for up to 1 hour as indicated. Some samples included 100μM GM6001. Control samples were activated with 10μM phorbol myristate acetate (PMA). Surface levels of (C) P-selectin (anti–P-selectin IgG) and (D) active αIIbβ3 (OG-fibrinogen binding) were assessed by flow cytometry, and (E) sGPVI levels in supernatant fractions were assessed by ELISA as described in “Measuring sGPVI.”
FXa induces GPVI shedding in washed platelets. Washed platelets resuspended at 5 × 108/mL in Tyrode buffer had no treatment (NT) or were mixed with (A) 0.1 μg/mL CVX, 10 μg/mL purified FX or FXa (10 μg/mL purified FX plus a 1:1 molar ratio of RVV to generate FXa) or (B) 5mM NEM or 10 μg/mL purified FXa. GM6001 (100μM) was added to platelets before addition of other factors where indicated. Platelet lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-GPVI cytoplasmic tail IgG or anti-GPV cytoplasmic tail IgG (B bottom panel), and visualized using chemiluminescence. A vertical line indicates a repositioned gel. Washed platelets (107/mL in Tyrode buffer) were pretreated with 10μM PP1 or PP2, 20μM BAPTA-AM, 1 μg/mL rivaroxaban (RIVA), or 10μM PAR1 inhibitor SCH79797 and then mixed with 10 μg/mL purified FXa for up to 1 hour as indicated. Some samples included 100μM GM6001. Control samples were activated with 10μM phorbol myristate acetate (PMA). Surface levels of (C) P-selectin (anti–P-selectin IgG) and (D) active αIIbβ3 (OG-fibrinogen binding) were assessed by flow cytometry, and (E) sGPVI levels in supernatant fractions were assessed by ELISA as described in “Measuring sGPVI.”
sGPVI in plasma from patients with DIC
If activation of coagulation induces shedding of platelet GPVI in vitro, it might be expected that patients with increased procoagulant activity could have increased plasma sGPVI in vivo. As an initial test of this concept, we investigated sGPVI levels in plasma from patients with DIC, a disease characterized by activation of the coagulation cascade resulting from different causes. DIC presents clinically with intravascular clot formation and ischemia of multiple organs, accompanied by consumption of different clotting factors and increased bleeding tendency, and typically with elevated bleeding time (including elevated APTT, prothrombin time, and international normalized ratio) and elevation of procoagulant markers, such as fibrinogen-derived D-dimer, and reduced fibrinogen levels.40,41 In 159 random community-based controls, mean values of sGPVI were 19.7 ± 8.1 ng/mL independent of age or gender.42 Analysis of sGPVI in 29 patients with DIC showed that plasma sGPVI was 53.9 ng/mL (95% confidence interval, 39.9-72.8 ng/mL) compared with 12.5 ng/mL (95% confidence interval, 9.0-17.3 ng/mL) levels in 36 chemotherapy- and/or cancer-related thrombocytopenic controls, and 14.6 ng/mL (95% confidence interval, 7.9-27 ng/mL) in 25 healthy controls (Table 1). Post-hoc testing for multiple comparisons showed that DIC patients were significantly different compared with thrombocytopenia controls (P < .0001) or healthy controls (P = .002). Age and platelet count were significantly different between DIC patients, thrombocytopenia controls, and healthy controls (P < .0001), whereas coagulation markers were significantly elevated in DIC patients compared with thrombocytopenic controls (P < .001). sGPVI remained significantly elevated in DIC patients (P < .0001) compared with thrombocytopenic controls after adjustment for age, platelet count, underlying cause, and coagulation markers (Table 1). Furthermore, sGPVI showed a positive correlation with APTT and international normalized ratio (r = 0.35, P = .003 and r = 0.68, P < .0001, respectively) but negatively correlated with fibrinogen (r = −0.62, P < .0001) using the Spearman rank correlation. Together, this suggests that sGPVI is correlated with enhanced coagulation in these patients.
Discussion
The aim of this study was to evaluate shedding of the platelet collagen receptor, GPVI in human plasma. Whereas previous studies from our laboratory and others have shown that collagen and other GPVI ligands induce metalloproteinase-mediated ectodomain shedding of GPVI, generating an approximately 55-kDa soluble fragment (sGPVI) and an approximately 10-kDa platelet-associated remnant fragment, here we show that coagulation of PRP also induces GPVI shedding in the absence of collagen or other known GPVI ligands. This was demonstrated in samples from healthy persons, where coagulation in the absence or presence of added TF significantly increased sGPVI in an FXa-dependent manner. A role for FXa, rather than thrombin or other coagulation factors, in regulating GPVI shedding was shown using (1) inhibitors that target FXa (enoxaparin or rivaroxaban) compared with thrombin inhibitors (dabigatran or hirudin); (2) RVV that directly activates FX (here shedding was only weakly inhibited by hirudin but completely inhibited by enoxaparin); and (3) FX-deficient plasma, where coagulation-induced GPVI shedding from washed platelets was impaired but restored by adding purified FX back to the plasma. Purified FXa, but not FX, also induced GPVI shedding from washed platelets, independently of platelet activation. FXa-induced GPVI shedding was metalloproteinase-mediated because it was strongly inhibited by the broad specificity metalloproteinase inhibitor GM6001 and at least partially blocked by a more selective ADAM10 inhibitor (GI254023).
A major role for FXa and a relatively minor role for thrombin or thrombin receptors in GPVI shedding from platelets in plasma are consistent with previous reports showing that thrombin is a weak inducer of GPVI shedding in washed platelets.16,17 FXa has previously been reported to activate the thrombin receptor PAR1,39 but the lack of GPVI shedding induced by either thrombin or the PAR1 agonist, thrombin receptor agonist peptide,36 and lack of inhibition of FXa-induced GPVI shedding by PP2 or BAPTA-AM, argues against FXa activating GPVI-shedding activity via PAR1. However, the mechanism for how FXa induces metalloproteinase-mediated shedding of platelet GPVI is yet to be determined. Although our results do not exclude the possibility that other metalloproteinases could also play some role, results in Figure 2 showing that coagulation-induced shedding is significantly inhibited by the ADAM10-selective inhibitor, GI25402343 at 2μM strongly support a key role for ADAM10 in this process. This is consistent with a selective role for ADAM10 (but not ADAM17) in proteolysis of peptides spanning the sheddase cleavage site in human GPVI.21 It is therefore possible that FXa directly activates ADAM10. In addition, at least some shedding of another platelet receptor, GPV, was also induced by FXa, and synthetic peptides based on extracellular sequences of both GPVI and GPV are cleaved by ADAM10.21 Thrombin-dependent platelet activation via GPIbα is enhanced when GPV is removed,44 and this could also be an additional functional consequence of FXa-induced GPV shedding. ADAM10 is a disintegrin and metalloproteinase family member consisting of a prodomain, a catalytic domain, a Cys-rich domain, transmembrane domain, and cytoplasmic tail. FXa has been reported to activate another metalloproteinase, matrix metalloproteinase-2 on human muscle cells, and converts pro-matrix metalloproteinase-2 to matrix metalloproteinase-2 by proteolysis within the prodomain. This effect was blocked by a FXa inhibitor and metalloproteinase inhibitor (GM6001) but not by hirudin.45 Matrix metalloproteinase-2 is expressed on activated human platelets and acts as a sheddase for CD40 ligand46 but is not known to cleave GPVI. The prodomain of ADAM10 contains a consensus FXa cleavage site (I-E/D-G-R̂) conserved in prothrombin, and it is interesting to speculate whether FXa cleaving within the prodomain of ADAM10 could convert it to an active form capable of cleaving GPVI. We cannot currently test this possibility on human platelets because of lack of reagents. Partial, rather than complete, inhibition of FXa-dependent shedding by GI254023 may be related to recent studies by Bender et al, in which analysis of surface-expressed GPVI in platelets where ADAM10/ADAM17 activity is ablated suggests ADAM17 or other metalloproteinases may also shed GPVI in mice.20
Some of the previously reported inhibitory effects of masking GPVI on collagen-induced or TF-dependent thrombin generation, thrombus formation, or pulmonary thromboembolism9-11 might be explained by loss of GPVI-mediated phosphatidylserine exposure, microparticle formation, secretion, or other procoagulant responses.14 However, none of these platelet responses would satisfactorily explain how GPVI blockade can inhibit TF-mediated coagulation in the absence of known GPVI ligands, suggesting additional interplay between platelet GPVI and coagulation factors. This is also suggested by the ability of GPVI ligands to induce dose-dependent increases in FXa and thrombin generation, regulated by a subpopulation of platelets with increased coagulation factor binding that is not correlated with levels of phosphatidylserine exposure.15 The ability of the activated coagulation cascade to induce GPVI shedding in the absence of GPVI ligands (this study) provides a mechanism for cross-regulation of platelet GPVI expression and coagulation, and a pathway for down-regulating GPVI under procoagulant conditions, independent of collagen exposure. As well as releasing sGPVI, FXa-induced shedding could irreversibly decrease GPVI expression and responsiveness to collagen and TF and limit the thrombotic propensity of platelets as a compensatory mechanism to prevent excessive thrombus formation under procoagulant conditions. The precise functional role of shed sGPVI in human subjects is unknown. A dimeric recombinant form of GPVI effectively inhibits GPVI-dependent thrombus formation in vivo in some experimental models,47,48 although it was ineffective in one report,49 possibly because of differences in collagen exposure. Monomeric GPVI ectodomain binds collagen and inhibits GPVI-dependent function in vitro but is significantly less potent than dimeric forms.50
In our study, rivaroxaban and enoxaparin completely block coagulation-induced GPVI shedding in vitro, whereas thrombin inhibitors dabigatran or hirudin only mildly inhibited shedding induced by activation of coagulation or by RVV. In contrast, all these inhibitors effectively block thrombin generation, and in work by other groups, both FXa inhibitors (rivaroxaban and apixaban) and FIIa inhibitors (dabigatran and argatroban) blocked TF-induced platelet aggregation involving active thrombin.51 Our findings imply that sGPVI could be elevated in plasma from patients with coagulopathy, such as DIC. Consistent with this possibility, we showed that plasma sGPVI levels were significantly higher in patients with DIC compared with levels in healthy community-based donors or hospitalized patients with thrombocytopenia but not DIC (Table 1). DIC can result from different underlying disorders, including sepsis, solid tumor, hematologic malignancies, severe trauma, and obstetric complications.40,41 In our cohort of DIC patients induced by sepsis (n = 13), hematologic malignancy (n = 5), solid tumor (n = 7), and other causes (n = 4, including multiple trauma, malaria, chronic liver disease, and blood transfusion incompatibility; Table 1), sGPVI was significantly higher in DIC patients compared with thrombocytopenic controls, regardless of underlying causes (P < .0001). sGPVI correlates positively with coagulation markers consistent with increased sGPVI release mediated by activated coagulation. These findings may have implications for the long-term clinical use of FXa or thrombin inhibitors for anticoagulation. Rivaroxaban and dabigatran are both promising oral drugs with predictable and reliable pharmacokinetic and pharmacodynamic profiles that have recently gained approval from European drug agencies for venous thromboembolism prophylaxis in orthopedic surgery.52-54 Dabigatran may be safer or more efficacious than warfarin in patients with atrial fibrillation.30 Our findings have potential impact where anticoagulants targeting FXa or FIIa or both are used for short- or long-term clinical use, that is, in orthopedic surgery or in atherothrombotic diseases related to myocardial infarction or ischemic stroke. Possible effects on platelet receptor shedding could have additional antithrombotic benefits or be a consideration if there are differential effects on expression of platelet receptors or other sheddase substrates. Plasma sGPVI is only derived from platelets and could provide a new readout for metalloproteinase activity in the context of coagulation, although plasma sGPVI may become elevated because of other causes, such as acute coronary syndrome, acute ischemic stroke, or autoimmune disease.35,42,55 Large-scale prospective studies would be required to evaluate sGPVI in the context of coagulation defects or anticoagulant treatments.
It remains to be established whether these drugs have comparable effects in vivo or whether effects on GPVI expression have additional unexpected antithrombotic/anticoagulant consequences by affecting GPVI-mediated collagen-induced platelet activation and procoagulant responses. Similarly, FXa and/or FXa/FIIa inhibitor regulation of the activity of ADAM10 or related metalloproteinases could potentially affect physiologic or pathologic processing of other ADAM10 substrates, notably amyloid precursor protein expressed on both platelets and nerve cells.56 Finally, increased GPVI shedding and plasma sGPVI could provide new therapeutic or diagnostic possibilities in other diseases leading to activation of FXa.39,57
In conclusion, coagulation-induced shedding of platelet GPVI in human plasma via a metalloproteinase-mediated FXa-dependent mechanism may down-regulate GPVI expression under procoagulant conditions independent of collagen. FXa inhibitors may have an additional role in modulating GPVI down-regulation via shedding, implying additional antithrombotic and anticoagulant consequences. The measurement of sGPVI could be a useful tool for understanding the interplay between platelet activation and activation of the coagulation cascade in patients with coagulopathy, such as DIC, or those treated with anticoagulants.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Hatem Salem and Prof Ross Baker for helpful discussions and Ms Jing Jing and Ms Margaret Collecutt for excellent assistance.
This work was supported by the National Health and Medical Research Council, Australia.
Authorship
Contribution: M.A.-T. designed and performed research, analyzed and interpreted data, and cowrote the manuscript; G.G., H.T., P.S., and M.C.B. designed research, analyzed and interpreted data, and contributed vital tools or reagents; E.P. analyzed and interpreted the data; and E.E.G. and R.K.A. supervised the study, designed research, analyzed and interpreted data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert K. Andrews, Australian Centre for Blood Diseases, Monash University, Alfred Medical Research and Education Precinct, Commercial Rd, Melbourne, Australia; e-mail: rob.andrews@monash.edu.
References
Author notes
E.E.G. and R.K.A. contributed equally to this study.