Abstract
Patients with Fanconi anemia (FA) have a high risk of developing acute myeloid leukemia (AML). In this study, we attempted to identify cell-surface markers for leukemia-initiating cells in FA-AML patients. We found that the IL-3 receptor-α (IL-3Rα) is a promising candidate as an leukemia-initiating cell-specific antigen for FA-AML. Whereas IL-3Rα expression is undetectable on normal CD34+CD38− HSCs, it is overexpressed on CD34+CD38− cells from FA patients with AML. We examined the leukemia-initiating cell activity of IL-3Rα–positive FA-AML cells in a “humanized” FA xenotransplant model in which we separated AML cells into IL-3Rα–positive and IL-3Rα–negative CD34 fractions and transplanted them into irradiated recipient mice. In all 3 FA-AML samples, only IL-3Rα–positive cells showed significant levels of engraftment and developed leukemia in the recipient mice. The FA CD34+IL-3Rα+ blasts isolated from leukemic mice exhibited hypersensitivity to IL-3 deprivation and JAK2-STAT5 overactivation after IL-3 treatment. Finally, treatment of FA CD34+IL-3Rα+ blasts with an IL-3Rα–neutralizing antibody inhibited IL-3–mediated proliferation and STAT5 activation. These results demonstrate that IL-3Rα is a cell-surface marker present on FA-AML leukemia-initiating cells and may be a valuable therapeutic target.
Introduction
Acute myeloid leukemia (AML), a heterogeneous group of hematologic malignancies characterized by an accumulation of clonal myeloid progenitor cells that do not differentiate normally,1,2 comprises approximately 25% of childhood acute leukemias.3 The treatment of AML remains a challenge, and most AML patients will die of their disease within 1-2 years of diagnosis.4 Conventional chemotherapeutic agents have been successful to some degree in treating AML, but now appear to have reached their maximum potential. Even with high-dose chemotherapy, only 30%-40% of AML patients survive, which is due mainly to relapse of the disease.5 Recently, novel therapeutic strategies for AML have focused on immune-based therapy through monoclonal antibodies that target and destroy leukemic blasts via specific cell receptors.6,7 These therapies were designed with the aim of selectively killing malignant cells that express unique antigens while sparing normal cells.
One of the recent advances in the AML field is the postulation that AML arises from a rare population of leukemic stem cells (LSCs).8,9 Phenotypic and functional analyses show that LSCs reside in the CD34+CD38− compartment, the primitive stem/progenitor population that also contains normal HSCs.10 Further studies demonstrated that both normal HSCs and LSCs share the properties of quiescence and self-renewal.8-10 This relatively dormant property of LSCs may contribute to the pattern of remission and subsequent relapse that is typical of the response to cytotoxic chemotherapy in AML. Therefore, it is believed that although most AML blasts can be eradicated by cytotoxic therapy, LSCs may be resistant to killing by chemotherapeutic agents. Recent studies have suggested that several antigens, such as CD33, CD44, CD96, CD123, and CLL-1, are specifically expressed in AML LSCs but not in normal HSCs.11-17 Because it is believed that LSCs are the most relevant target population for novel antileukemic therapy, these unique antigens present opportunities for selectively targeting AML LSCs.
One of the best studied AML models is Fanconi anemia (FA), a genetic disorder associated with bone marrow failure, clonal proliferation of HSCs and progenitor cells, and progression to myelodysplastic syndrome (MDS) and AML.18-20 FA is caused by a deficiency in any of the 14 genes that encode the FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ/BRIP1, FANCL, FANCM, FANCN/PALB2, and FANCO/RAD51C proteins.21-24 The biologic function of FA proteins has been the subject of intense investigation in recent years.
One of the most important clinical features of FA is hematologic. Patients with FA often develop pancytopenia during their first few years of life. Complications of bone marrow failure (BMF) are the major causes of morbidity and mortality of FA: at least 80% of FA patients die from BMF.20,25 FA patients have a dramatically (> 300-fold) increased susceptibility of developing MDS or AML.19,20,26 It is known that FA patients frequently develop clonal chromosomal abnormalities in the bone marrow cells in the later stage of the disease.26 In fact, certain clonal cytogenetic abnormalities, such as 3q addition, 5q deletion, and monosomy 7, are common in MDS and AML, occurring secondary to treatment with chemotherapeutic agents and in children with FA who have evolved to MDS and AML.25-28
To better understand the biology of FA-AML, we performed immunophenotypic and functional analyses to identify its leukemia-initiating cell–specific antigen. We present results demonstrating that IL-3 receptor α (IL-3Rα/CD123) is a cell-surface marker present on leukemia-initiating cells of patients with FA-AML, and may be a promising therapeutic target for these patients.
Methods
Leukemia and normal bone marrow cells
Normal and FA bone marrow samples were obtained from the Cincinnati Children's Hospital Medical Center Fanconi Anemia Comprehensive Care Center in accordance with guidelines from their institutional review board (protocol #2008-1644).
Cell separation, immunophenotyping, and cell sorting
Bone marrow mononuclear cells were isolated by Ficoll (GE Healthcare) gradient centrifugation and cryopreserved for later use. CD34+ normal or AML bone marrow cells were enriched using the MACS CD34 isolation kit (Miltenyi Biotec). The isolated CD34+ cells were expanded in serum-free medium (StemSpan SFEM; Stemcell Biotech) supplemented with a human cytokine cocktail containing recombinant human SCF, Flt-3, IL-3, and IL-6 (StemSpan CC100; Stemcell Biotech) for 3 or 5 days. Single-cell suspensions were washed with PBS containing 2% fetal calf serum, incubated with 10% human AB serum for 20 minutes to prevent nonspecific antibody binding, and stained with FITC-CD34, allophycocyanin-CD38 (APC-CD38), PE-CD33, PE-CD44, PE-CD96, PE-CD123 (IL-3Rα), or PE-CLL-1 (BD Pharmingen), respectively, for 30 minutes on ice. Cells were then washed and resuspended in 300 μL of PBS followed by flow cytometric analysis using an FACSCanto (BD Biosciences). Data analysis was done with FACSDiva Version 6.1.2 software (BD Biosciences) or FlowJo Version 5.7.2 software (TreeStar). For cell sorting, CD34+IL-3Rα+ and CD34+IL-3Rα− populations were isolated by incubating the 5-day-cultured CD34+ cells with FITC-CD34 and PE–IL-3Rα antibodies (BD Pharmingen), followed by cell sorting using the FACSAria II (BD Biosciences). For isolation of CD45+IL-3Rα+ FA-AML blast cells from the bone marrow of leukemic mice, cells were stained with FITC-CD45 and PE-IL-3Rα antibodies (BD Pharmingen), followed by cell sorting using the FACSAria II (BD Biosciences).
Bone marrow transplantation and establishment of NSG/SMG3 xenografts
Age-matched NSG (NOD scid IL-2Rγ−/−) or NSG/SGM3 (NOD scid IL-2Rγ−/−/SCF, GM-CSF, and IL-3) mice (provided by Dr Jim Mulloy, Cincinnati Children's Hospital Medical Center) were used as transplant recipients. These mice were sublethally irradiated (2.5-2.8 Gy, 110 cGy/min, 137Cs). Sorted CD34+IL-3Rα+ and CD34+IL-3Rα− cells from 3 FA complementation group A (FA-A) AML patients or normal CD34+ bone marrow cells were transplanted directly into the right femurs of sublethally irradiated NSG/SGM3 recipient mice. Six weeks later, bone marrow cells from recipient mice were aspirated and subjected to flow cytometric analysis by staining with anti–human CD45 (APC), CD34 (FITC), and IL-3Rα (PE) antibodies (BD Pharmingen) for donor engraftment. Fifteen weeks after transplantation, bone marrow cells were harvested and transplanted into 3 secondary NSG/SGM3 or NSG recipient mice to determine leukemic development. Donor-engrafted populations were measured by staining the aspirated bone marrow samples with anti–human CD 45 antibody (BD Pharmingen).
Cell culture, in vitro treatment, and cell-proliferation assay
CD45+IL-3Rα+ cells from the bone marrow of leukemic mice were isolated by FACS and cultured in the presence of 10 ng/mL of IL-3 (R&D Systems) or treated with the IL-3–neutralizing antibody 7G3 (BD Pharmingen) or the isotype-matched control antibody IgG2a (BD Pharmingen) for 15 minutes in vitro. For recombinant human GM-CSF (PeproTech) induction, 5 ng/mL was used. Cell proliferation was measured by BrdU incorporation assay using the APC BrdU Flow Kit (BD Pharmingen). In brief, cells were cultured for 45 minutes in normal growth medium supplemented with 10μM BrdU, harvested, fixed in Cytofix/Cytoperm buffer (BD Pharmingen), treated with Cytoperm Plus Buffer (BD Pharmingen) on ice, and then more Cytofix/Cytoperm buffer was added for refixation. The cells were incubated with DNase to expose incorporated BrdU and then stained with APC-conjugated antibody specific for BrdU. Cells were then washed and counterstained with 30 μL of 7-amino-actinomycin D (7AAD) per sample. The percentages of BrdU-positive cells were determined using the FACSCanto (BD Biosciences).
Apoptosis assay
CD45+IL-3Rα+ cells from the bone marrow of leukemic mice were grown in the presence of 10 ng/mL of IL-3 for 2 days, shifted to IL-3–free medium for an additional 2 days, harvested for immunostaining using antibody specific for APC-conjugated annexin V and 7AAD (BD Biosciences), and analyzed by flow cytometry. Annexin V–positive populations were determined as apoptotic cells using the FACSCanto (BD Biosciences).
Preparation of cell extracts, immunoblotting, and immunoprecipitation
To prepare whole-cell extracts, cells were washed with ice-cold PBS, and resuspended in ice-cold lysis buffer containing 50mM Tris-HCL (pH 7.4), 0.1% NP40, and 1M NaCl supplemented with protease and phosphatase inhibitors (10 μg/mL of aprotinin, 25 μg/mL of leupeptin, 10 μg/mL of pepstatin A, 2mM PMSF, 0.1M NaP2O4, 25mM NaF, and 2mM sodium orthovanadate) for 30 minutes on ice. Cell debris was removed from the lysates by centrifuging them at 16873g for 30 minutes. Protein concentration was quantified using BioRad reagent. Approximately 100 μg of total protein was resolved on SDS-PAGE (7.5%) and transferred onto nitrocellulose membranes. Immunoblots were then incubated with primary antibodies specific for JAK2, G410 (Upstate Biotech), STAT5a (Santa Cruz Biotechnology), STAT5a (Tyr 697; Cell Signaling Technology), or β-actin (Sigma-Aldrich) for 12-16 hours at 4°C. Signals were visualized by incubation with anti-mouse or anti-rabbit secondary antibodies, followed by ECL chemiluminescence (Amersham Biosciences). For immunoprecipitation, cells were lysed in NP-40 lysis buffer (20mM Tris, pH 8.0, 137mM NaCl, 10% glycerol, 1% NP-40, 0.15 U/mL of aprotinin, 1mM PMSF, 20μM leupeptin, and 5mM sodium vanadate). Whole-cell extracts (0.5-1 mg of total proteins) were incubated with the antibody specific for JAK2 (Upstate Biotech) or isotype-matched control antibodies for 2 hours at 4°C. Beads were washed 3 times and immunocomplexes were collected. The samples were examined directly by SDS-PAGE and immunoblotting using phosphor-tyrosine antibody 4G10 (Upstate Biotech).
Results
Overexpression of IL-3Rα on CD34+CD38− cells from FA patients with AML
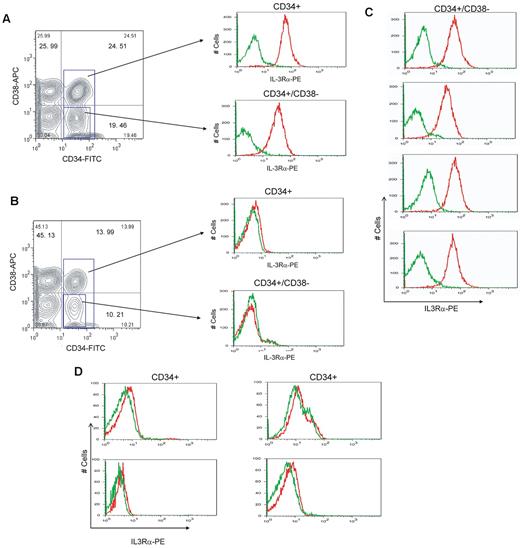
Recent studies have suggested that several antigens, such as CD33, CD44, CD96, IL-3Rα, and CLL-1, are specifically expressed in AML LSCs but not in normal HSCs.11-17 To better understand the biology of FA-AML, we first made an effort to identify and isolate primitive leukemic cells from the bone marrow of FA-AML patients. Because it is known that LSCs are present in the CD34+CD38− compartment,29 we performed flow cytometric studies using CD34+-enriched bone marrow cells from normal donors and FA-AML patients (Table 1). We found that CD33 and CD44 was expressed on normal CD34 cells and on FA-AML cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article; and Table 1), and that CD96 and CLL-1 staining was very weak, probably due to technical difficulties (data not shown). On the other hand, IL-3Rα was highly expressed in both the overall CD34+ population and in the more primitive CD34+CD38− compartment purified from a FA-AML patient carrying a mutation in the FA-A gene (FANCA; Figure 1A). In contrast, IL-3Rα expression was very low or undetectable in the total CD34+ population and in the more primitive CD34+CD38− cells of normal bone marrow (Figure 1B; Table 1). Moreover, IL-3Rα staining on CD34+CD38− cells of 4 additional FA-AML samples (3 FA-A patients and 1 FA-C patient; Table 1) demonstrated the high level of IL-3Rα expression in FA leukemic populations (Figure 1C). Interestingly, the expression levels of IL-3Rα on CD34+ cells from 3 FA patients with BMF (Table 1) were either undetectable or very low (Figure 1D), suggesting that the phenotypic overexpression of IL-3Rα may have been acquired during leukemic evolution.
IL-3Rα expression on FA leukemic cells and normal CD34+ cells. (A) Low-density bone marrow cells from an FA-A AML patient were enriched for CD34+ cells by selection on an immunoaffinity column (Miltenyi Biotec), expanded in stem-cell medium for 3 days, and subjected to flow cytometric analysis. The gates indicate the total CD34+ population and the CD34+CD38− population. The histograms indicate the IL-3Rα labeling for the 2 gated populations. (B) The same analysis as in panel A but for the CD34-enriched bone marrow cells from a normal donor. (C) IL-3Rα expression on 4 additional FA-AML samples. Four primary FA-AML specimens were labeled with antibodies for CD34, CD38, and IL-3Rα and analyzed by flow cytometry. (D) The same analysis as in panel C but for the CD34+ bone marrow cells from 4 FA patients with BMF. The histograms show IL-3Rα labeling for the CD34+CD38− or CD34+ gated populations from each sample. The red sections indicate IL-3Rα staining and the green sections indicate parallel labeling with an isotype control antibody. For each sample, 50 000 events were analyzed.
IL-3Rα expression on FA leukemic cells and normal CD34+ cells. (A) Low-density bone marrow cells from an FA-A AML patient were enriched for CD34+ cells by selection on an immunoaffinity column (Miltenyi Biotec), expanded in stem-cell medium for 3 days, and subjected to flow cytometric analysis. The gates indicate the total CD34+ population and the CD34+CD38− population. The histograms indicate the IL-3Rα labeling for the 2 gated populations. (B) The same analysis as in panel A but for the CD34-enriched bone marrow cells from a normal donor. (C) IL-3Rα expression on 4 additional FA-AML samples. Four primary FA-AML specimens were labeled with antibodies for CD34, CD38, and IL-3Rα and analyzed by flow cytometry. (D) The same analysis as in panel C but for the CD34+ bone marrow cells from 4 FA patients with BMF. The histograms show IL-3Rα labeling for the CD34+CD38− or CD34+ gated populations from each sample. The red sections indicate IL-3Rα staining and the green sections indicate parallel labeling with an isotype control antibody. For each sample, 50 000 events were analyzed.
Leukemia-initiating cell activity of IL-3Rα–positive FA-AML cells in a “humanized” xenotransplant model
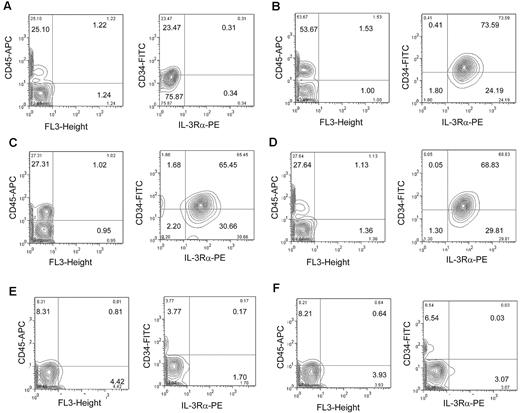
To establish the LSC functional capacity of IL-3Rα–positive FA-AML cells, we performed transplantation studies using the NSG/SGM3 xenotransplantation model. These recipient mice express transgenic cDNAs encoding human SCF, GM-CSF, and IL-3. Three primary FA-A AML specimens were flow cytometrically sorted for CD34+IL-3Rα+ and CD34+IL-3Rα− cells, which were transplanted directly into the right femurs of sublethally irradiated NSG/SGM3 recipient mice. The intrafemoral approach circumvents the AML LSC trafficking/homing processes associated with the circulation.7 We also transplanted normal bone marrow CD34+ cells in parallel. After 6 weeks, samples were obtained by bone marrow aspiration and analyzed for engraftment by flow cytometry. The results showed that both normal CD34+ cells and FA-AML CD34+ IL-3Rα+ cells could successfully engraft (human CD45+) in the marrow of NSG/SGM3 mice (Figure 2A-D left panels). Further analysis indicated that the progeny derived from normal HSCs did not express IL-3Rα (Figure 2A right panel). In contrast, 65%-73% of the human CD45+ gated cells (CD34+ and CD34− subsets) derived from the 3 FA-AML patients, which had proliferated in vivo, were IL-3Rα+ (Figure 2B-D right panels). We observed very low donor engraftment in recipient mice transplanted with CD34+IL-3Rα− cells from FA-AML patient samples (Figure 2E-F).
Repopulating ability of FA-AML LSCs in NSG/SGM3 mice. Flow cytometry of engrafted human cells in NSG/SGM3 mice. 1-3 × 105 FACS-sorted CD34+ cells from a normal donor (A) and CD34+IL-3Rα+ (B-D) or CD34+IL-3Rα− cells (E-F) from FA-AML patients were injected into the right femurs of sublethally irradiated NSG/SGM3 recipient mice. Six weeks after transplantation, bone marrow cells were isolated and analyzed for the engraftment of human cells using human anti-CD45 antibody (left panels). The right panels show the CD34 compared with the IL-3Rα profiles of the human CD45+ gated population. For each sample, 50 000-100 000 events were analyzed.
Repopulating ability of FA-AML LSCs in NSG/SGM3 mice. Flow cytometry of engrafted human cells in NSG/SGM3 mice. 1-3 × 105 FACS-sorted CD34+ cells from a normal donor (A) and CD34+IL-3Rα+ (B-D) or CD34+IL-3Rα− cells (E-F) from FA-AML patients were injected into the right femurs of sublethally irradiated NSG/SGM3 recipient mice. Six weeks after transplantation, bone marrow cells were isolated and analyzed for the engraftment of human cells using human anti-CD45 antibody (left panels). The right panels show the CD34 compared with the IL-3Rα profiles of the human CD45+ gated population. For each sample, 50 000-100 000 events were analyzed.
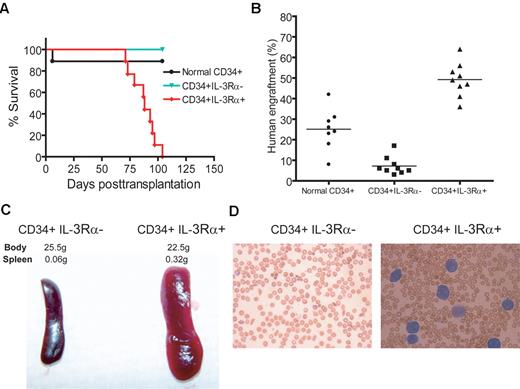
To determine the activity of FA-AML LSCs, we performed secondary transplantation by injecting 7-10 × 106 cells isolated from the bone marrow of each primary recipient mouse into secondary recipient mice to determine leukemic development. Within 15 weeks after transplantation, all 9 secondary recipient mice receiving cells from primary recipient mice transplanted with CD34+IL-3Rα+ FA-AML cells developed leukemia (Figure 3A). In contrast, the secondary recipients of CD34+IL-3Rα− cells or normal CD34+ cells survived without signs of leukemia for 4 months after transplantation, which might have been due to poor engraftment (Figure 3B). The IL-3Rα+ FA-AML–derived leukemic mice showed massive splenomegaly (Figure 3C) and increased human myeloblasts in the peripheral blood (Figure 3D). These results confirmed our previous phenotypic identification of the CD34+CD38−IL-3Rα+ subset as the potential LSCs associated with FA-AML. Therefore, because of the results from the immunophenotypic analysis and the NSG/SGM3 FA xenotransplant model, we concluded that IL-3Rα is specifically expressed on FA-AML LSCs.
CD34+IL-3Rα+ FA-AML cells give rise to leukemia in secondary recipient mice. (A) Cells (7-10 × 106) isolated from the bone marrow of the primary mice transplanted with normal CD34+ cells, or CD34+ IL-3Rα− or CD34+IL-3Rα+ FA-AML cells were injected intrafemorally into each NSG/SGM3 or NSG recipient mouse (n = 9 for each group). The survival of recipient mice was analyzed with a Kaplan-Meier plot. (B) Assessment of xenografts in the bone marrow of secondary recipient mice was performed by flow cytometric analysis of human CD45 staining. Each symbol represents a single mouse, and horizontal bars indicate the mean value. Note that 1 recipient mouse transplanted with normal donor cells died 6 days after transplantation, probably due to irradiation. (C-D) Representative images of spleens (C) and Giemsa-stained peripheral blood smears (D) of CD34+IL-3Rα− or CD34+IL-3Rα+ secondary recipient mice.
CD34+IL-3Rα+ FA-AML cells give rise to leukemia in secondary recipient mice. (A) Cells (7-10 × 106) isolated from the bone marrow of the primary mice transplanted with normal CD34+ cells, or CD34+ IL-3Rα− or CD34+IL-3Rα+ FA-AML cells were injected intrafemorally into each NSG/SGM3 or NSG recipient mouse (n = 9 for each group). The survival of recipient mice was analyzed with a Kaplan-Meier plot. (B) Assessment of xenografts in the bone marrow of secondary recipient mice was performed by flow cytometric analysis of human CD45 staining. Each symbol represents a single mouse, and horizontal bars indicate the mean value. Note that 1 recipient mouse transplanted with normal donor cells died 6 days after transplantation, probably due to irradiation. (C-D) Representative images of spleens (C) and Giemsa-stained peripheral blood smears (D) of CD34+IL-3Rα− or CD34+IL-3Rα+ secondary recipient mice.
High IL-3 responsiveness of FA IL-3Rα–positive blasts
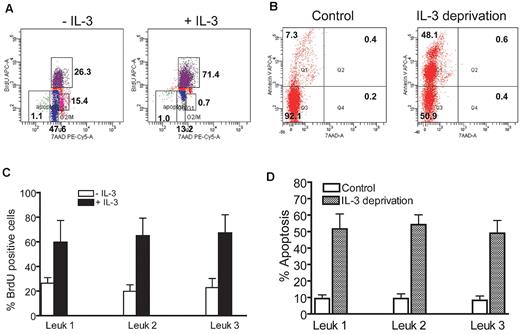
To evaluate the biologic response of the progeny derived from FA-AML LSCs, we isolated human CD45+IL-3Rα+ cells from the bone marrow of leukemic mice and determined IL-3–stimulated proliferation and apoptosis after IL-3 withdrawal. These analyses, performed on sorted CD45+IL-3Rα+ cells from the bone marrow of 3 leukemic mice receiving CD34+IL-3Rα+ cells from 3 respective FA-A AML patients, showed that the FA IL-3Rα–positive blasts displayed a significantly higher proliferative rate in the presence of IL-3 than untreated control cells (Figure 4A,C). We next examined whether IL-3 deprivation could induce apoptosis of the FA IL-3Rα–positive blasts. In these experiments, leukemic blasts were grown in the presence of IL-3 for 2 days and then shifted to IL-3 free medium for an additional 2 days. Leukemic cells grown in IL-3–deprived medium exhibited a significant increase in the percentage of apoptotic cells compared with control cells without IL-3 deprivation (Figure 4B,D). These results indicate a role for IL-3Rα in FA-AML blast proliferation and survival.
High responsiveness of FA IL-3Rα–positive blasts to IL-3. (A) FA IL-3Rα–positive blasts displayed a significantly higher proliferative rate in the presence of IL-3. Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic mice were cultured in the presence of IL-3 (10 ng/mL) for 15 minutes, followed by BrdU incorporation assay. (B) Cells described in panel A were continuously cultured in the presence of 10 ng/mL of IL-3 for 2 days, and then shifted to IL-3 free medium for an additional 2 days. Cell apoptosis was then determined by annexin V/7AAD double staining, followed by flow cytometric analysis. (C-D) Quantitative analysis of BrdU incorporation and apoptosis. Data are presented as the percentage of BrdU incorporated or of apoptotic cells. Results of individual samples were plotted after normalization with total cell number. Results are means ± SD of 3 independent experiments.
High responsiveness of FA IL-3Rα–positive blasts to IL-3. (A) FA IL-3Rα–positive blasts displayed a significantly higher proliferative rate in the presence of IL-3. Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic mice were cultured in the presence of IL-3 (10 ng/mL) for 15 minutes, followed by BrdU incorporation assay. (B) Cells described in panel A were continuously cultured in the presence of 10 ng/mL of IL-3 for 2 days, and then shifted to IL-3 free medium for an additional 2 days. Cell apoptosis was then determined by annexin V/7AAD double staining, followed by flow cytometric analysis. (C-D) Quantitative analysis of BrdU incorporation and apoptosis. Data are presented as the percentage of BrdU incorporated or of apoptotic cells. Results of individual samples were plotted after normalization with total cell number. Results are means ± SD of 3 independent experiments.
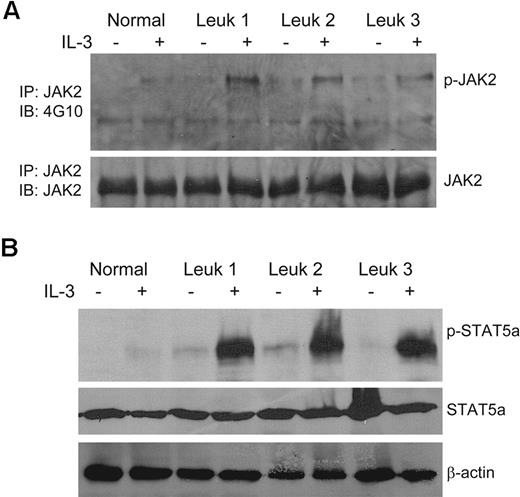
FA IL-3Rα–positive blasts exhibit JAK2/STAT5 overactivation in response to IL-3
Stimulation of AML cells with IL-3 leads to several well-characterized intracellular signal transduction events, including activation of the JAK2/STAT5 signaling pathway.30,31 Therefore, we performed biochemical analysis to evaluate the degree of phosphorylation of JAK2 and STAT5, which are the key transcription factors in IL-3 signaling, in FA CD45+IL-3Rα+ cells from the bone marrow of leukemic mice. We observed low levels of constitutive activation of JAK2 (Figure 5A) and STAT5a (Figure 5B) in all 3 samples studied in the absence of IL-3 stimulation. However, IL-3 stimulation greatly increased the phosphorylation of both JAK2 and STAT5a in FA IL-3Rα–positive blasts compared with normal controls. These results indicate that high IL-3Rα expression in FA-AML cells correlates with elevated IL-3 responsiveness.
Overactivation of JAK2/STAT5 in FA IL-3Rα–positive blasts in response to IL-3. (A) Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic recipient mice were isolated by FACS, cultured in the presence or absence of IL-3 (10 ng/mL) for 15 minutes, and whole-cell extracts were prepared. Total proteins (0.5-1 mg) for each indicated sample were subjected to immunoprecipitation with anti-JAK2 antibody. The immune complexes were resolved on 7.5% SDS-PAGE, transferred to nylon membrane, and probed with the anti-phosphotyrosine mAb 4G10 or an anti-JAK2 antibody. (B) Direct Western blot for STAT5a. Whole-cell extracts (∼ 100 μg of total proteins for each indicated sample) from the cells described in panel A were subjected to SDS-PAGE and immunoblotted with antibodies specific for phosphor-STAT5a, total STAT5a, or β-actin.
Overactivation of JAK2/STAT5 in FA IL-3Rα–positive blasts in response to IL-3. (A) Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic recipient mice were isolated by FACS, cultured in the presence or absence of IL-3 (10 ng/mL) for 15 minutes, and whole-cell extracts were prepared. Total proteins (0.5-1 mg) for each indicated sample were subjected to immunoprecipitation with anti-JAK2 antibody. The immune complexes were resolved on 7.5% SDS-PAGE, transferred to nylon membrane, and probed with the anti-phosphotyrosine mAb 4G10 or an anti-JAK2 antibody. (B) Direct Western blot for STAT5a. Whole-cell extracts (∼ 100 μg of total proteins for each indicated sample) from the cells described in panel A were subjected to SDS-PAGE and immunoblotted with antibodies specific for phosphor-STAT5a, total STAT5a, or β-actin.
Treatment of FA IL-3Rα+ cells with an IL-3Rα–neutralizing antibody inhibits IL-3–mediated proliferation and STAT5 activation
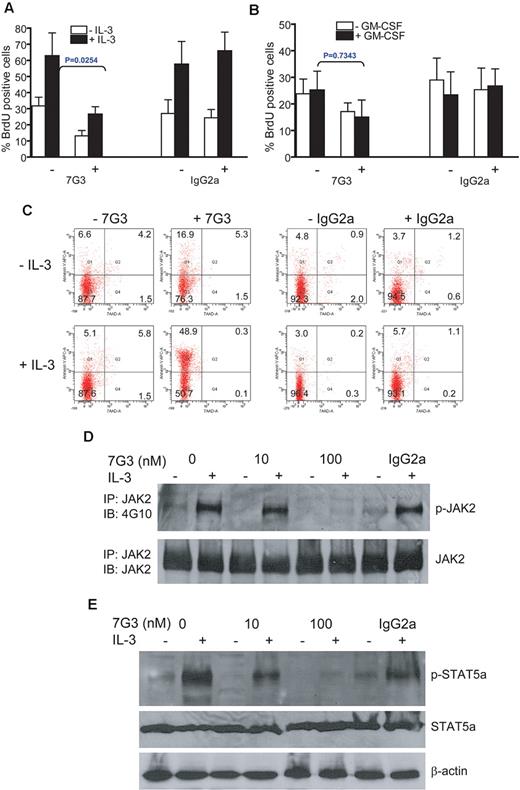
The observations that FA-AML cells express high levels of IL-3Rα and exhibit elevated IL-3 responsiveness prompted us to assess whether blocking IL-3 signaling could eliminate the FA IL-3Rα+ AML blasts. We treated FA CD45+IL-3Rα+ cells isolated from the bone marrow of leukemic mice with the IL-3Rα–neutralizing antibody 7G3, which has previously been shown to inhibit IL-3–induced proliferation in AML cells,13 and then determined proliferation of the cells using an assay that measures BrdU incorporation. As shown in Figure 6A, treatment of FA IL-3Rα+ AML cells with 7G3 reduced IL-3–induced BrdU incorporation by approximately 50% compared with treatment with the isotype antibody IgG2a. In addition, 7G3 inhibited the proliferation of the cells, albeit less profoundly, in the absence of exogenous IL-3 (Figure 6A). This is consistent with the observation of a low level of constitutive activation of IL-3 signaling in these FA IL-3Rα+ AML cells (Figure 5). This inhibition by 7G3 was IL-3–specific, because 7G3 had no effect on GM-CSF–induced cell proliferation (Figure 6B). We also examined whether 7G3 treatment induced apoptosis of the CD45+IL-3Rα+ cells isolated from leukemic recipient mice. In these experiments, the sorted CD45+IL-3Rα+ leukemic blasts treated with or without IL-3 were grown in the presence of 7G3 or the matched isotype antibody IgG2a for 6 hours, followed by annexin V/7AAD staining. We found that leukemic cells grown in 7G3 medium exhibited a significant increase in the percentage of apoptotic cells compared with those incubated with the control isotype antibody (Figure 6C).
Pretreatment of FA IL-3Rα+ blasts with the IL-3Rα–neutralizing antibody inhibits IL-3–mediated proliferation and STAT5 activation. (A) Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic recipient mice were isolated by FACS and cultured in the IL-3–neutralizing Ab 7G3 (100nM) or the isotype-matched antibody IgG2a (100nM) for 6 hours in the absence or presence of IL-3 (10 ng/mL), followed by BrdU incorporation assay. (B) Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic recipient mice were isolated by FACS and cultured in the IL-3–neutralizing Ab 7G3 (100nM) or the isotype-matched antibody IgG2a (100nM) for 6 hours in the absence or presence of GM-CSF (5 ng/mL), followed by BrdU incorporation assay. P value indicates comparison of difference between 7G3-treated and -untreated groups. (C) Cells described in panel B were cultured in the IL-3–neutralizing Ab 7G3 (100nM) or the isotype-matched antibody IgG2a (100nM) for 6 hours. Apoptosis was determined by Annexin V/7AAD double staining, followed by flow cytometric analysis. (D) Whole-cell extracts were prepared from the cells described in panel A, followed by immunoprecipitation with the anti-JAK2 antibody. The immune complexes were resolved on 7.5% SDS-PAGE, transferred to nylon membrane, and probed with anti-JAK2 or anti-phosphotyrosine mAb 4G10. (E) Whole-cell extracts from cells described in panel A were subjected to SDS-PAGE and immunoblotted with antibodies specific for phosphor-STAT5a, total STAT5a, or β-actin.
Pretreatment of FA IL-3Rα+ blasts with the IL-3Rα–neutralizing antibody inhibits IL-3–mediated proliferation and STAT5 activation. (A) Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic recipient mice were isolated by FACS and cultured in the IL-3–neutralizing Ab 7G3 (100nM) or the isotype-matched antibody IgG2a (100nM) for 6 hours in the absence or presence of IL-3 (10 ng/mL), followed by BrdU incorporation assay. (B) Human CD45+IL-3Rα+ cells from the bone marrow of FA leukemic recipient mice were isolated by FACS and cultured in the IL-3–neutralizing Ab 7G3 (100nM) or the isotype-matched antibody IgG2a (100nM) for 6 hours in the absence or presence of GM-CSF (5 ng/mL), followed by BrdU incorporation assay. P value indicates comparison of difference between 7G3-treated and -untreated groups. (C) Cells described in panel B were cultured in the IL-3–neutralizing Ab 7G3 (100nM) or the isotype-matched antibody IgG2a (100nM) for 6 hours. Apoptosis was determined by Annexin V/7AAD double staining, followed by flow cytometric analysis. (D) Whole-cell extracts were prepared from the cells described in panel A, followed by immunoprecipitation with the anti-JAK2 antibody. The immune complexes were resolved on 7.5% SDS-PAGE, transferred to nylon membrane, and probed with anti-JAK2 or anti-phosphotyrosine mAb 4G10. (E) Whole-cell extracts from cells described in panel A were subjected to SDS-PAGE and immunoblotted with antibodies specific for phosphor-STAT5a, total STAT5a, or β-actin.
Because the FA IL-3Rα+ AML cells showed elevated IL-3 responsiveness, we next investigated whether 7G3 inhibited cell proliferation by blocking IL-3–mediated signaling. Because we observed overactivation of both JAK2 and STAT5a in FA IL-3Rα–positive blasts treated with IL-3, we examined the effect of 7G3 on JAK2 and STAT5a phosphorylation. Strikingly, 7G3 inhibited IL-3–induced phosphorylation of both JAK2 (Figure 6D) and STAT5a (Figure 6E) in FA IL-3Rα–positive blasts in a dose-dependent manner. These results indicate that anti-IL-3Rα neutralization inhibits FA IL-3Rα–positive cell proliferation by blocking IL-3–mediated signaling.
Discussion
FA has been proposed as a genetic model system for studying hematologic malignancies, because the disease commonly progresses from BMF to a preleukemic myelodysplastic syndrome stage, and finally evolves exclusively to AML.18,25 In FA-AML, overall survival rates remain below 30%, and the long-term outcome is dismal.19,32 Furthermore, current treatment strategies are associated with significant morbidity and mortality from intensive chemotherapy and HSC transplantation protocols. Emphasis is being placed on developing novel therapies targeting the specific genetic alterations involved in leukemia. One remaining challenge, however, is to eradicate the residue LSCs that often lead to disease relapse.33 To this end, one emerging concept in combinatory therapy is that the relatively quiescent LSCs can be “chased out of” the protective bone marrow niche by one means while being effectively targeted for eradication by conventional chemotherapy.34-36 In the present study, we attempted to identify unique markers for leukemia-initiating cells in FA-AML patients and found that IL-3Rα is selectively overexpressed on CD34+CD38− cells from FA patients with AML. Using a “humanized” FA xenotransplant model, we demonstrated that only IL-3Rα+ cells showed significant levels of engraftment and developed leukemia in secondary recipient mice. We further demonstrate that the FA CD34+IL-3Rα+ blasts isolated from the leukemic mice exhibited hypersensitivity to IL-3 deprivation and JAK2/STAT5 overactivation after IL-3 stimulation. Treatment of FA CD34+IL-3Rα+ blasts with an IL-3Rα–neutralizing antibody inhibited IL-3–mediated proliferation and JAK2/STAT5 activation. These results suggest that IL-3Rα is a promising cell-surface marker present on FA-AML leukemia-initiating cells that may serve as a potential therapeutic target for FA-AML treatment.
IL-3Rα is selectively overexpressed on CD34+CD38− cells from FA patients with AML, but not in the total CD34+ population or in more primitive CD34+CD38− cells of normal bone marrow (Figure 1). Outside of FA-AML populations, many recent studies have also demonstrated that IL-3Rα is highly expressed on AML blasts, CD34+ leukemic progenitors, and AML-LSCs.15,16,30,31,37-40 However, our study has several notable features that distinguish it from some of these studies. First, to our knowledge, this is the first report that identifies a potential LSC marker in the FA-AML population. Second, our study establishes an FA-AML xenotransplant model using the “humanized” NSG/SGM3 mice. These “humanized” mice have proven to be better recipients of human cells than NOD/SCID mice by us and by others. With this robust model, we were able to show that the CD34+ IL-3Rα+ subset from FA-AML patients possessed leukemia-initiating cell activity and gave rise to leukemia in secondary recipient mice (Figure 3). Third, we demonstrated that CD34+ cells from 3 FA patients with BMF did not overexpress IL-3Rα, suggesting that the phenotypic overexpression of IL-3Rα may have been acquired during leukemic evolution. In this context, our finding provides a unique tool not only to separate putative FA-AML LSCs from their progeny, but also to separate leukemic and nonleukemic stem cells from the same patient, thus allowing further investigation into the function and interaction between the 2 populations.
Another notable observation of the present study was that the FA IL-3Rα–positive blasts isolated from leukemic mice displayed a significantly higher proliferative rate in response to IL-3 stimulation than unstimulated cells (Figure 4), and this was correlated with IL-3–dependent hyperactivation of the JAK2/STAT5 pathway (Figure 5). Overexpression of IL-3Rα on AML cells has been reported to confer a growth advantage over normal HSCs. Indeed, tissue-culture experiments have shown that AML cells proliferate extensively in the presence of IL-3,41-44 and primary AML samples have been found to secrete cytokines, including IL-3.45-47 Mechanistically, high proliferation of IL-3Rα–overexpressing AML cells is positively correlated with the level of IL-3–stimulated and spontaneous STAT5 activation.31,43,44 In this context, because IL-3 signaling is strongly JAK2/STAT5 dependent, we believe that FA-AML cells share this common pathway, as our data suggest (Figures 5–6). In the clinical setting, high IL-3Rα expression in AML is associated with higher blast counts at diagnosis and a lower remission rate that results in reduced survival.31,48,49 Therefore, IL-3Rα expression in FA-AML LSCs may be a molecular feature linked to poor prognosis in FA-AML patients.
Overexpression of IL-3Rα on LSCs presents an opportunity for selectively targeting AML LSCs with immunotherapeutic strategies. It has been reported that a monoclonal antibody to IL-3Rα, 7G3, could inhibit IL-3–mediated proliferation of leukemic cell lines and primary AML blasts.7,50 Encouraged by these observations, we investigated the effect of 7G3 on FA CD34+IL-3Rα+ blasts isolated from leukemic mice and found that the IL-3Rα–neutralizing antibody inhibited IL-3–mediated proliferation and JAK2/STAT5 activation (Figure 6). These results are consistent with a recent study demonstrating that the 7G3 antibody effectively suppresses non–FA-AML cells.7 Because 7G3 specifically blocks IL-3 signaling, and because both the FA-AML samples and the non–FA-AML samples used in that recent study express high levels of CD123 expression, it is not surprising that FA-AML cells react to this neutralizing antibody as effectively as non–FA-AML cells. In addition, the coincidence of overexpression of IL-3Rα with leukemia-initiating cell activity has important implications for FA-AML chemotherapy and HSC transplantation. The identification of IL-3Rα as a potential FA-AML leukemia-initiating cell marker may aid in developing novel strategies for successful chemotherapy or stem cell therapy in FA-AML patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Jim Mulloy (Cincinnati Children's Hospital Medical Center) for NSG/SGM3 mice and discussions, the Fanconi Anemia Comprehensive Care Center at Cincinnati Children's Hospital Medical Center for bone marrow samples, and Jeff Bailey and Victoria Summey (Cincinnati Children's Hospital Medical Center Comprehensive Mouse and Cancer Core) for technical assistance in bone marrow transplantation.
This work was supported in part by a Leukemia Research Foundation grant and NIH grants R01 CA109641 and R01 HL076712. Q.P. is supported by a Leukemia & Lymphoma Society scholar award. W.D. is supported by a Fanconi Anemia Research Fund grant.
National Institutes of Health
Authorship
Contribution: W.D. designed and performed research, analyzed data, and wrote the paper; X.L. performed research and analyzed data; J.S. performed research; and Q.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qishen Pang or Wei Du, Division of Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: qishen.pang@cchmc.org or wei.du@cchmc.org.