ITP has served as a model for autoimmune disorders with disturbances of the innate and adaptive immunity where targeted treatment with immunomodulation has proven effective. In this issue of Blood, Zhang et al report that these immune disturbances are triggered by oxydative stress.1 In addition, the molecular-based results indicate the possibility of distinguishing the transient, self-limited form of ITP from chronic, long-term ITP.
Confronted with a patient with newly diagnosed ITP, the physician cannot determine if the patient has a transient, self-limited disorder or long-term, chronic ITP. In children ITP is often present after an infection or vaccination. In adults, ITP is associated with heliobacter pylory, hepatits C virus, HIV, and other viral infections, although the mechanism is not clear.2,3 It is unknown how platelets are targeted by the host's immune system. Infection-related oxidative stress may induce disturbed immune response. (Auto-)antibodies or immune complexes against platelets lead to early destruction of platelets by phagocytosis or by cytotoxic T cells4 in predisposed individuals. The immune disturbances of ITP and of other autoimmune disorders have been indirectly documented by therapeutic immunomodulatory intervention such as intravenous human immunoglobulin concentrate, which targets the whole immune response,5 monoclonal anti-CD20 antibodies,6 by cyclosporine A, or by nonspecific immunosuppressants on an empiric basis.7,8
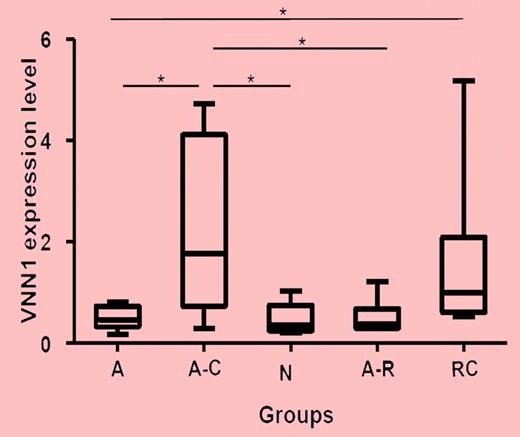
Real-time PCR validation of VNN1 expression in different ITP groups and healthy controls. Five groups of samples were included in the validation: self-limited acute ITP (A; n = 8), chronic acute to chronic ITP during the acute phase (A-C; n = 7), healthy control (n, n = 5), resolved acute ITP (A-R; n = 6), and chronic ITP resistant to multiple treatments (RC; n = 6). The nonparametric Mann-Whitney 2-tailed test was performed in the statistical analysis. At the transcriptional level, VNN1 expression in the A-C group is significantly higher compared with the A (P = .0093), N (P = .0177), and A-R (P = .0221) groups; VNN1 expression in the RC group is significantly higher than in the A group (P = .0127). The upper and lower limits of each box represent the 75th and 25th percentiles, respectively; the horizontal lines inside the boxes represent medians; and the whiskers, extreme measurements.
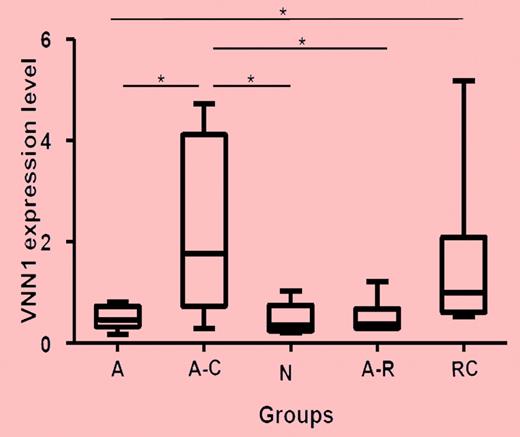
Real-time PCR validation of VNN1 expression in different ITP groups and healthy controls. Five groups of samples were included in the validation: self-limited acute ITP (A; n = 8), chronic acute to chronic ITP during the acute phase (A-C; n = 7), healthy control (n, n = 5), resolved acute ITP (A-R; n = 6), and chronic ITP resistant to multiple treatments (RC; n = 6). The nonparametric Mann-Whitney 2-tailed test was performed in the statistical analysis. At the transcriptional level, VNN1 expression in the A-C group is significantly higher compared with the A (P = .0093), N (P = .0177), and A-R (P = .0221) groups; VNN1 expression in the RC group is significantly higher than in the A group (P = .0127). The upper and lower limits of each box represent the 75th and 25th percentiles, respectively; the horizontal lines inside the boxes represent medians; and the whiskers, extreme measurements.
Zhang and colleagues report here on gene-expression and molecular-oxidative stress results as causative factors for chronic ITP in children.1 With transcriptome cDNA microarray analysis of peripheral blood, the authors could show differences of clustering profiles among patients with transient, self-limited ITP and chronic, long-term ITP and control individuals. Overexpression of the gene vanin-1 (VNN1)—an oxidative stress sensor—was associated with chronic ITP only (see figure). VNN1 is characterized by its role in oxidative stress response, and it mediates production of inflammatory cytokines by antagonizing peroxisome proliferative-activated receptor γ (PPARγ). VNN1 is the only gene that was detected in chronic ITP. Exposure of human blood mononuclear cells to oxidative stress inducers (LPS, sodium arsenite) up-regulates VNN1. Quantitative real-time PCR measurement of VNN1 expression confirmed the oxidative stress events in peripheral blood cells. In addition, the ratio of reduced to oxidized gluthation—a parameter of the cellular redox state—was significantly down-modulated in children with chronic ITP in comparison to healthy controls.
In the present study by Zhang et al, the numbers of the patient/control groups are small, but ongoing oxygen stress is a significant factor in patients with chronic ITP. Chronic ITP is an autoimmune disorder where isolated low-platelet counts indicate the immune pathogenesis. In chronic ITP, clinical bleedings occur in some patients with very low platelet counts. Many other autoimmune disorders such as Guillain Barré syndrome, Kawasaki syndrome, lupus erythematosus, dermatomyositis, and dermatologic blistering disease have similar pathogeneses and respond to similar therapeutic interventions.5 The demonstrated pathway should now be confirmed by a larger study including adults with ITP as well as in other autoimmune disorders.
From these new findings early prognostic estimation concerning transient or long-term disease may be possible. The pathophysiologic changes of the involved molecules in oxidative stress could create new therapeutic approaches and medications. The described triggering pathways of chronic autoimmune phenomena might provide earlier intervention in selected groups of an autoimmune disorder.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■