Abstract
A transient erythromyeloid wave of definitive hematopoietic progenitors (erythroid/myeloid progenitors [EMPs]) emerges in the yolk sac beginning at embryonic day 8.25 (E8.25) and colonizes the liver by E10.5, before adult-repopulating hematopoietic stem cells. At E11.5, we observe all maturational stages of erythroid precursors in the liver and the first definitive erythrocytes in the circulation. These early fetal liver erythroblasts express predominantly adult β-globins and the definitive erythroid-specific transcriptional modifiers c-myb, Sox6, and Bcl11A. Surprisingly, they also express low levels of “embryonic” βH1-, but not εy-, globin transcripts. Consistent with these results, RNA polymerase and highly modified histones are found associated with βH1- and adult globin, but not εy-globin, genes. E11.5 definitive proerythroblasts from mice transgenic for the human β-globin locus, like human fetal erythroblasts, express predominately human γ-, low β-, and no ε-globin transcripts. Significantly, E9.5 yolk sac–derived EMPs cultured in vitro have similar murine and human transgenic globin expression patterns. Later liver proerythroblasts express low levels of γ-globin, while adult marrow proerythroblasts express only β-globin transcripts. We conclude that yolk sac–derived EMPs, the first of 2 origins of definitive erythropoiesis, express a unique pattern of globin genes as they generate the first definitive erythrocytes in the liver of the mammalian embryo.
Introduction
In the adult, steady-state erythropoiesis begins with hematopoietic stem cells (HSCs) in the bone marrow (BM) that differentiate into lineage-restricted erythroid progenitors. These progenitors subsequently generate morphologically identifiable precursors that mature into enucleated red blood cells (RBCs). Embryonic erythropoiesis differs from the adult in 2 critical ways. First, it is not in steady state, as exponentially increasing numbers of RBCs must be generated to meet the needs of the rapidly growing embryo.1 Second, RBCs are required for embryonic survival before transplantable HSCs are formed. In the mouse, circulating erythroid cells are required for normal development beyond embryonic day 9.5 (E9.5).2 However, the first adult-repopulating HSCs are not observed until E10.5 and have just begun to colonize the site of fetal hematopoiesis, the forming liver, between E11.5 and E12.5.3-5
One solution for this embryonic dilemma, the appearance of 2 distinct forms of erythroid cells in the bloodstream of mammalian embryos, was described over 100 years ago.6 The first form consisted of “primitive” erythroid cells that emerge from the yolk sac and were characterized by their large size and nucleated state. Primitive erythroid cells were superseded in the circulation by a second erythroid cell population, which was termed “definitive,” because of their resemblance to the smaller enucleated RBCs found in the adult. More recent studies have determined that the primitive erythroid lineage arises from a transient wave of primitive erythroid progenitors (EryP-CFC) present in the yolk sac between E7.25-E9.0.7,8 These progenitors generate a semisynchronous wave of erythroblasts that mature in the circulation.1 Later, HSC expansion in the fetal liver between E12.5 and E16.54 is associated with an exponential increase in the number of definitive erythroid progenitors and the production of massive numbers of definitive erythroid precursors exclusively expressing adult globins.9,10 Thus, HSCs that colonize the fetal liver are commonly described as the origin of “definitive” hematopoiesis in the mammalian embryo.
However, a closer examination of “definitive” erythropoiesis indicates that this model is an oversimplification of the early ontogeny of mammalian hematopoiesis. Analysis of blood smears from mouse embryos indicates that enucleated definitive RBCs first enter the bloodstream between E11.5 and E12.5.1,11 Thus, the entrance of mature definitive reticulocytes into the bloodstream coincides temporally with the initial colonization of the fetal liver by HSC. What then is the developmental origin of these first “definitive” erythrocytes? Before its colonization by HSC, the liver at E10.5 contains over a thousand definitive erythroid progenitors that subsequently expand over 10-fold in number by E11.5.3-5,8 Several lines of evidence support the concept that the source of these definitive erythroid progenitors in the early fetal liver is a wave of definitive erythroid/myeloid potential arising in the yolk sac at E8.25. This wave of hematopoietic potential is composed of definitive erythroid (BFU-E), megakaryocyte, as well as several myeloid lineages, including multipotential HPP-CFC progenitors.7,8,12,13 These progenitors expand in numbers within the yolk sac and enter the circulation by E9.0 after the onset of cardiac function.8 Definitive hematopoietic progenitors have also been detected in the embryo proper and placenta soon after embryonic circulation has started.8,14 As definitive hematopoietic progenitors are present in circulating blood, it is currently not known if these other tissues generate EMPs de novo. In circulation-defective embryos, normal numbers of definitive hematopoietic progenitors emerge in the yolk sac but remain confined there and are not found in the embryo proper.15 Between E9.0 and E10.5, the aorta-gonad-mesonephros (AGM) becomes enriched in HSCs, but not BFU-E, while most embryonic BFU-E and myeloid progenitors are concentrated in the circulation and then the fetal liver.8,16 In the zebrafish, fate mapping studies of intact embryos demonstrate a similar transient wave of erythroid/myeloid progenitors (EMPs) arising after primitive hematopoiesis and distinct from HSC formation. EMP-derived cells colonize the fetal kidney and mature into the first definitive blood cells.17 Thus, the term EMP has been used to distinguish this transient wave of hematopoietic progenitors from earlier primitive hematopoiesis and from later HSC-derived hematopoiesis.
Here, we examined the early ontogeny of definitive erythropoiesis in the mouse embryo. We report the appearance at E11.5 of enucleated definitive RBCs in the circulation and the presence of all stages of erythroid intermediates in the liver before HSC colonization. We found that erythroid precursors in the early fetal liver express a previously undescribed pattern of globin genes associated with a distinct chromatin landscape at the β-globin locus. In addition, in mice transgenic for the human β-globin locus, the first definitive erythroid precursors predominately express γ-globin. E9.5 yolk-sac cells exhibit the same unique β-globin expression and high transgenic γ-globin expression when cultured in vitro. These findings support the concept that EMP-derived hematopoietic progenitors are the source of the first definitive erythroid cells to differentiate in the fetal liver. Our studies indicate that “definitive” erythropoiesis has 2 distinct developmental origins and patterns of globin gene expression in the mammalian embryo.
Methods
Tissue collection and processing
Outbred (CD1) Swiss Webster mice (Taconic) and human β-globin locus transgenic mice18 were maintained on 12-hour dark and light cycles. Mice were mated overnight and vaginal plugs examined in the morning (E0.3). At specified times during gestation, mice were killed by CO2 inhalation. Embryonic tissues were dissected in PB2 (DPBS; Invitrogen) with 0.1 g/L CaCl2, 0.3% BSA (Gemini Bio-Products; 0.1% glucose). Yolk sac and embryo proper tissues were isolated from staged embryos and dissociated as previously described.8 Fetal blood was collected as previously described.1 Embryonic livers and adult marrow were dissociated by gentle pipetting in PB2. The University of Rochester Committee on Animal Resources approved all animal experiments.
Imaging flow cytometry and cell sorting
Single-cell suspensions stained with Abs (Ter119-APC, c-kit–PE, CD41-FITC or CD71-FITC; eBioscience) and 5 μg/mL DAPI (Invitrogen) were sorted on a FACS Aria (BD Biosciences). Cells stained with Ter119-biotin and streptavidin PE–Texas Red, c-kit–PE (eBioscience), 2 μg/mL thiazol orange (Sigma-Aldrich), and 10μM DRAQ5 (Biostatus) were run on the ImageStream (Amnis) and erythroid intermediates were analyzed with IDEAS software (Amnis) as previously published.19 Rare maternal RBC contamination was eliminated based on thiazol orange negativity. Primitive and fetal definitive erythroid cells were distinguished using Ter119 and brightfield image intensities (Figure 1). The classification of primitive erythroid cells by this approach was confirmed previously by combining immunohistochemistry with Abs to εy-globin with imaging flow cytometry.20
Methylcelluose culture and in vitro erythropoiesis assays
Erythroid colony assays were performed as previously described.21 Yolk sac and fetal liver BFU-E could be detected at 2-3 days by their faint red color. Marking colonies at day 3 and observing their complete maturation at day 7, confirmed that these early pink colonies were nascent BFU-E. In vitro erythroid maturation was carried out in a 2-step culture system. Sorted c-kit+, CD41hi progenitors from E9.5 yolk sacs were cultured in IMDM, 10% serum replacement, 10% protein free hybridoma II medium (Invitrogen), 5% Stem Cell Accelerator serum (Animal Technologies), 2mM glutamine (Invitrogen), 0.15mM MTG (Sigma-Aldrich), 2 U/mL erythropoietin (Amgen), and 100 ng/mL SCF (PeproTech). After 24 hours, nonadherent cells were transferred and cultured in the same media without SCF.
Real-time quantitative PCR
RNA was extracted from sorted and cultured cells using RNeasy Plus Mini Kits according to the manufacturer's instructions (QIAGEN), and cDNA synthesized using SuperScript First-Strand Synthesis Systems (Invitrogen). Individual erythroid colonies were plucked from methylcellulose, washed in 0.5 mL of PBS, and cDNA made the using the “cells to CT” kit according to the manufacturer's instructions (Applied Biosystems). Only immature erythroid colonies with low β1-globin mRNA levels (0.2%-4% of 18S RNA levels) and free of primitive erythroid cell contamination, as determined by little or no εy-globin message, were included. Colonies were categorized as positive for βH1-globin message if it was > 0.1% of the β1-globin message detected. Quantitative PCR (qPCR) was performed with the primers found in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) with either with TaqMan system (Applied Biosystems) or SYBR Green (Quanta BioScience). All expression levels were normalized to 18S message levels in the same cDNA. Negative control cDNA reactions made without reverse transcriptase were used for each sample and gene analyzed.
In situ hybridization
Analysis of β1-, εy-, and βH1-globin transcript accumulation was performed as previously described22 using gene-specific in vitro–transcribed RNA probes internally labeled with 33P. Darkfield and brightfield images were obtained with an Optiphot microscope (Nikon) using 10× (NA0.85) objectives at room temperature and a SPOT RT-Slider digital camera (Diagnostic Instruments). Images were acquired, processed, pseudocolored, and combined using Photoshop (Adobe) with Fovea Pro (Reindeer Graphics) plugins.
ChIP
Cells were formaldehyde cross-linked, sonicated, and immunoprecipitated, and DNA isolated as previously described.22 An “input” fraction was removed to serve as a control. Abs used for immunoprecipitation were normal rabbit IgG (Upstate-Millipore) as a control; antihistone H3 acetylated at K9 and/or K16 (Upstate-Millipore); anti-histone H3 dimethylated at K4 (Upstate-Millipore and Abcam); and anti-RNA polymerase II (Santa Cruz Biotechnology). Analysis was performed using qPCR with RNA isolated using TRIzol reagent (Invitrogen) according to the manufacturer's recommendations. A total of 1 μg of RNA was used to synthesize cDNA using the iScript cDNA synthesis kit (Bio-Rad). Primers were designed to amplify regions within the murine β-globin locus. Regions within loci that are inactive in erythroid cells served as controls, including amylase, protamine, and TCR β-chain, as well as an intergenic region located 8.2 kb 3′ of the εy-globin transcription start site. Primers and enrichment calculation are as previously reported.22
Results
Definitive erythrocytes enter the circulation by E11.5
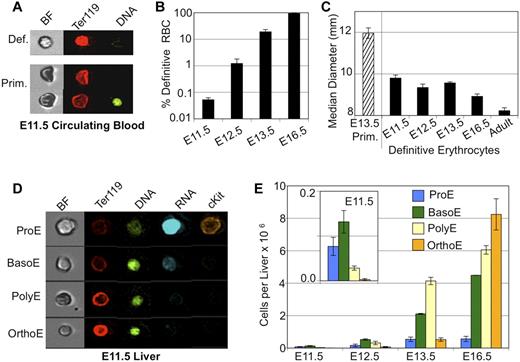
There is considerable temporal overlap in the appearance of primitive and definitive erythrocytes in the bloodstream of the mouse embryo.1,11 To more carefully define the emergence of definitive RBCs, we used imaging flow cytometry to distinguish primitive and definitive erythrocytes. Primitive erythroid cells exhibit lower Ter119 staining and darker brightfield characteristics (Figure 1A). As shown in Figure 1, panels B and A, the small number of definitive erythrocytes were consistently detected in the bloodstream of E11.5 mouse embryos. This population of definitive erythrocytes made up < 0.1% of the total number of circulating cells at E11.5. Over the next few days, the output of definitive RBCs quickly increased, so that by E13.5 they constituted ∼ 20% of the circulating erythroid cells and by E16.5 are > 94% of all circulating cells. The early definitive erythrocytes are similar in size to later fetal definitive RBCs, both of which are significantly smaller than enucleated primitive erythroid cells but larger than adult RBCs (Figure 1C).
Onset of definitive erythropoiesis by E11.5. (A) Imaging flow cytometry of E11.5 blood. Definitive RBCs (top) have higher mean levels of Ter119 staining and lighter brightfield intensity then cocirculating primitive erythroid cells (bottom). (B) Percent of total erythroid (Ter119-positive) cells that are definitive in fetal blood are plotted by embryonic day (note log scale). Low but consistent numbers of definitive erythroid cells are seen by E11.5. (C) Median diameter of definitive erythroid cells demonstrates early definitive RBC size is consistent with later fetal RBC size and different from primitive or adult definitive erythroid cells. (D) Imaging flow cytometry demonstrated all stages of definitive erythroblasts in the E11.5 liver. Maturational stages were determined by combined morphologic and fluorescent parameters including cell and nuclear size, RNA (thiazole orange) staining and mean intensity of Ter119 and DNA (DRAQ5) staining. (E) Absolute numbers of staged erythroblasts per liver during fetal development. Inset is a smaller scale plot of E11.5 data. Error bars are SEM, n = 3 or 4.
Onset of definitive erythropoiesis by E11.5. (A) Imaging flow cytometry of E11.5 blood. Definitive RBCs (top) have higher mean levels of Ter119 staining and lighter brightfield intensity then cocirculating primitive erythroid cells (bottom). (B) Percent of total erythroid (Ter119-positive) cells that are definitive in fetal blood are plotted by embryonic day (note log scale). Low but consistent numbers of definitive erythroid cells are seen by E11.5. (C) Median diameter of definitive erythroid cells demonstrates early definitive RBC size is consistent with later fetal RBC size and different from primitive or adult definitive erythroid cells. (D) Imaging flow cytometry demonstrated all stages of definitive erythroblasts in the E11.5 liver. Maturational stages were determined by combined morphologic and fluorescent parameters including cell and nuclear size, RNA (thiazole orange) staining and mean intensity of Ter119 and DNA (DRAQ5) staining. (E) Absolute numbers of staged erythroblasts per liver during fetal development. Inset is a smaller scale plot of E11.5 data. Error bars are SEM, n = 3 or 4.
Definitive erythropoiesis begins in the liver by E11.5, before HSC colonization
If the definitive hematopoietic progenitors that colonize the emerging fetal liver are the source of the first definitive RBCs entering the bloodstream at E11.5, then there should be evidence of the intermediate erythroid precursors maturing in the E11.5 liver. The onset of erythropoiesis in the early liver has been analyzed by flow cytometry with Ter119 and CD71 Abs. However, this approach was problematic because of significant presence at these early time points of circulating primitive erythroid cells that also express these cell-surface markers (supplemental Figure 1). Therefore, imaging flow cytometry was used to identify and quantify definitive erythroid precursors. We had previously used this experimental approach, which combines data of nuclear and cellular size along with fluorescent stains, to quantify progressive stages of erythroblast maturation in the BM.19 Nucleated primitive erythroblasts could be excluded from analysis based on their characteristic large size, low mean Ter119 staining, and small bright nucleus (Figure 1A,D). Using this strategy, we identified all stages (pro-, basophilic, polychromatophilic, and orthochromatic) of maturing definitive erythroblasts in the fetal liver at E11.5 (Figure 1D-E). The less mature proerythroblasts and basophilic erythroblasts were more prevalent at this time point, consistent with the onset of definitive erythropoiesis. By E16.5, the more mature polychromatophilic and orthochromatic erythroblasts predominate, similar to what is seen in the adult BM (supplemental Figure 1).19 We also found a 190-fold expansion in the number of maturing definitive erythroblasts in the fetal liver between E11.5 and E16.5 as the fetal liver produces increasing numbers of erythrocytes for the growing embryo (supplemental Figure 1).
“Embryonic” βH1-globin is expressed in the fetal liver during the onset of definitive erythropoiesis
We had previously examined the spatial and temporal expression of “embryonic” (εy, βH1) and “adult” (β1, β2) β-globin genes in primitive erythroid cells as they mature in the circulation of the murine embryo.22 Primitive erythroblasts in the bloodstream at E12.5 contain high levels of εy-globin transcripts and low levels of βH1-globin transcripts, while definitive cells maturing in the liver express high levels of β1-globin (Figure 2). Unexpectedly, we also found regions of βH1-globin expressing cells within the liver parenchyma at E11.5 and E12.5 (Figure 2 and data not shown). While primitive erythroid cells migrate into the liver to complete enucleation,23,24 the βH1-globin expression observed in the fetal liver was significantly higher than in the circulating primitive erythroblasts. Furthermore, the lack of a similar pattern of εy-globin expression in adjacent tissue sections suggested the possibility that βH1-globin was expressed by maturing definitive erythroid precursors. In situ hybridization studies of the fetal liver at later developmental time points revealed only adult β-globin expression (data not shown), indicating that βH1-globin was specifically expressed in the early fetal liver.
βH1-globin, but not εy-globin, transcripts are present in the early fetal liver. Neighboring sections of E12.5 liver were hybridized to radioactive probes (exposed grains indicating hybridized probe are colorized red). β1-globin is expressed predominantly in liver (fl) cells, while εy-globin is expressed in circulating primitive erythroid cells (fb). βH1-globin is expressed in low levels in primitive erythroid cells as expected, but also more highly expressed in a subset of cells in the liver. Size bar equals 100 μm. Images were processed as described in “In situ hybridization.”
βH1-globin, but not εy-globin, transcripts are present in the early fetal liver. Neighboring sections of E12.5 liver were hybridized to radioactive probes (exposed grains indicating hybridized probe are colorized red). β1-globin is expressed predominantly in liver (fl) cells, while εy-globin is expressed in circulating primitive erythroid cells (fb). βH1-globin is expressed in low levels in primitive erythroid cells as expected, but also more highly expressed in a subset of cells in the liver. Size bar equals 100 μm. Images were processed as described in “In situ hybridization.”
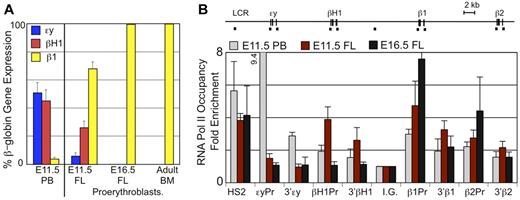
To better characterize globin expression in definitive erythroid cells within the fetal liver, it was necessary to separate them from primitive erythroid cells which contain much higher globin transcript levels than the less mature definitive erythroid precursors in the liver. By E11.5, the synchronously maturing primitive erythroblasts are predominantly polychromatophilic erythroblasts and no longer express c-kit while the least mature definitive erythroblasts are c-kit positive (Figure 1D and supplemental Figure 2). Therefore, we isolated Ter119lo, c-kit+ definitive proerythroblasts in the fetal liver by flow cytometry and examined their globin expression by qPCR (Figure 3A). Definitive proerythroblasts in the E11.5 liver express significant levels of βH1-globin transcripts, although less than adult β1-globin transcripts. These levels of βH1-globin transcripts cannot be accounted for by contamination with primitive erythroid cells as they are not accompanied by equivalent levels of embryonic εy-globin transcripts present in E11.5 primitive erythroid cells (Figure 3A). We also detected trace amounts of εy-globin transcripts but cannot distinguish if this expression is because of rare contamination with primitive erythroblasts or to extremely low level of transcription of this gene in the definitive proerythroblasts. Proerythroblasts isolated from late fetal stages or the adult BM do not express embryonic globins, indicating that the βH1-globin expression is unique to definitive erythroid cells found in the early fetal liver (Figure 3A).
βH1-globin expression in fetal liver definitive erythroid cells. (A) Levels of εy-, βH1-, and β1-globin transcripts were determined by qPCR and graphed as a percentage of total β-globin transcripts. Circulating E11.5 primitive erythroid cells (PB) express high levels of εy- and βH1-globin. Proerythroblasts (Ter119lo, c-kit+) from late fetal liver and adult BM express β1-globin and no embryonic globins. While E11.5 liver proerythroblasts express a unique combination of β-globins with both βH1- and β1- but little if any εy-globin. Error bars are SEM of 3 samples. (B) RNA Pol II occupancy at the β-globin locus was determined by ChIP. The murine β-globin locus is depicted to scale above the graph with the probes used indicated by short dashes. Significantly higher levels of RNA Pol II were found associated the βH1-globin promoter than the εy-globin promoter in E11.5 fetal liver cells (P < .007) based on 1-way ANOVA analysis. Error bars represent SEM of 3 samples.
βH1-globin expression in fetal liver definitive erythroid cells. (A) Levels of εy-, βH1-, and β1-globin transcripts were determined by qPCR and graphed as a percentage of total β-globin transcripts. Circulating E11.5 primitive erythroid cells (PB) express high levels of εy- and βH1-globin. Proerythroblasts (Ter119lo, c-kit+) from late fetal liver and adult BM express β1-globin and no embryonic globins. While E11.5 liver proerythroblasts express a unique combination of β-globins with both βH1- and β1- but little if any εy-globin. Error bars are SEM of 3 samples. (B) RNA Pol II occupancy at the β-globin locus was determined by ChIP. The murine β-globin locus is depicted to scale above the graph with the probes used indicated by short dashes. Significantly higher levels of RNA Pol II were found associated the βH1-globin promoter than the εy-globin promoter in E11.5 fetal liver cells (P < .007) based on 1-way ANOVA analysis. Error bars represent SEM of 3 samples.
To further investigate whether definitive erythroid precursors in the E11.5 fetal liver transcribe βH1-globin, we examined RNA polymerase II (RNA Pol II) occupancy of the β-globin genes using chromatin immunoprecipitation. We found that E11.5 fetal liver cells have significant RNA Pol II occupancy of the βH1-globin promoter as well as the β1- and β2-globin promoters, but not the εy-globin promoter (Figure 3B). This is in contrast to the primitive erythroid cells isolated from E11.5 peripheral blood which had highest levels of RNA Pol II bound to the εy-globin promoter with lower levels seen at the other β-globin genes. In addition, in later fetal liver (E16.5), RNA polymerase is only associated with the β1- and β2-globin genes, as previously published.22 The pattern of RNA Pol II binding is consistent with the unique globin expression in E11.5 proerythroblasts and provides further in vivo evidence that the βH1- and εy-globin genes are differentially regulated during ontogeny.
βH1-globin expression is associated with EMP-derived yolk-sac hematopoietic definitive progenitors
Given that the novel expression of βH1-globin in fetal definitive erythropoiesis precedes HSC colonization of the liver, we asked whether it might be uniquely associated with the emergence of the EMP wave of hematopoiesis in the yolk sac. Definitive hematopoietic progenitors were isolated from E9.5 yolk sac by flow cytometry based on c-kit and CD41 expression25,26 and cultured in a 2-step erythroid differentiation assay. Maturing erythroid cells up-regulated both β1- and βH1-globin to a similar degree after 2 days in culture, while εy-globin transcripts were barely detectable (Figure 4A). These results indicate that maturing definitive erythroid cells derived from the E9.5 yolk sac, before colonization of the fetal liver, have the same pattern of β-globin expression as found in the first definitive erythroblasts present in E11.5-E12.5 fetal liver.
βH1-globin expression is associated with yolk sac–derived definitive hematopoietic progenitors. (A) E9.5 yolk sac progenitors (c-kithi, CD41hi) cultured in a 2-step erythroid differentiation assay express βH1-globin as they mature and switch to predominantly β1-globin expression. SEM of 3 experiments is indicated by error bars. (B) Globin expression of cells derived from individual BFU-E–derived cultures expressing βH1-globin are only found in the yolk sac and early fetal liver. Pale red BFU-E colonies at day 2 to 4 methylcellulose cultures were analyzed individually by qPCR for expression of εy-, βH1-, and β1-globin. Proportions of colonies with βH1-globin expression over the total colonies analyzed are shown.
βH1-globin expression is associated with yolk sac–derived definitive hematopoietic progenitors. (A) E9.5 yolk sac progenitors (c-kithi, CD41hi) cultured in a 2-step erythroid differentiation assay express βH1-globin as they mature and switch to predominantly β1-globin expression. SEM of 3 experiments is indicated by error bars. (B) Globin expression of cells derived from individual BFU-E–derived cultures expressing βH1-globin are only found in the yolk sac and early fetal liver. Pale red BFU-E colonies at day 2 to 4 methylcellulose cultures were analyzed individually by qPCR for expression of εy-, βH1-, and β1-globin. Proportions of colonies with βH1-globin expression over the total colonies analyzed are shown.
The in situ hybridization analysis indicated that βH1-globin is expressed in a subset of erythroid cells (Figure 2). This could be because of the intermixing of 2 erythroid lineages, one of which expresses βH1-globin. Alternatively, βH1-globin expression may be restricted to a maturational subset of erythroid precursors, particularly immature precursors including proerythroblasts. Interestingly, we have previously identified a maturational βH1- to-εy-globin switch in primitive erythroid cells as they mature in vivo.22 Here we see a maturational βH1-to-β1-globin switch as definitive erythroid cells derived from the E9.5 yolk sac mature in vitro (Figure 4A). To determine whether the observed β-globin expression pattern represented a common characteristic of EMP-derived definitive erythroid progenitors, we analyzed globin gene expression in individual erythroid colonies from the E9.5 yolk sac. We found, as published previously, the exclusive presence of adult β-globin transcripts in the cells composing the fully mature erythroid colonies at day 7 of culture (data not shown).8 However, all nascent BFU-E–derived colonies from E9.5 yolk sac, when plucked at 2 to 4 days of in vitro culture, contained detectable levels of βH1-globin message (Figure 4B). We also found βH1-globin–expressing definitive BFU-E–derived colonies in the early fetal liver between E10.5 and E12.5. Subsequently, nascent BFU-E in the liver only express the adult globins (Figure 4B). Taken together, these data indicate that βH1-globin is transiently expressed as globin transcription is activated in EMP-derived definitive erythroid precursors.
E11.5 liver erythroid cells express definitive erythroid-specific transcription factors but have a unique chromatin domain at the β-globin locus
We had previously determined that c-myb and Sox6 are specifically expressed by definitive, but not primitive, erythroid cells.27,28 Recently, Bcl11A has also been found to be uniquely expressed in definitive erythroid cells and to regulate globin gene expression.29 In contrast, the GATA-1 and EKLF transcription factors function both in primitive and in definitive erythropoiesis.2,30-33 We examined the expression of these 5 transcription factors in erythroid precursors throughout ontogeny. As shown in Figure 5, GATA-1 and EKLF, but not c-myb, Sox6, or Bcl11A, are expressed in E11.5 primitive erythroblasts. In contrast, all 5 of these transcription factors are expressed in E11.5 liver proerythroblasts, as well as E16.5 liver and adult BM proerythroblasts. These data provide further evidence that EMP-derived E11.5 liver proerythroblasts are “definitive” in nature. However, the expression of Bcl11A and Sox6 in E11.5 liver proerythroblasts, that also express βH1-globin, is intriguing because these factors are thought to silence “embryonic” globin genes in both fetal and adult murine erythropoiesis.28,34
Early fetal liver erythropoiesis associated with c-myb, Sox6, and Bcl11A erythroid transcription factors. Expression of common erythroid transcription factors (GATA-1, EKLF) and those uniquely expressed in definitive erythroid lineages (c-myb, Sox6, and Bcl11A) in primitive erythroid cells from peripheral blood (PB) and in definitive proerythroblasts sorted from fetal liver (FL) and BM was determined by qPCR. Error bars indicate SEM and n = 3.
Early fetal liver erythropoiesis associated with c-myb, Sox6, and Bcl11A erythroid transcription factors. Expression of common erythroid transcription factors (GATA-1, EKLF) and those uniquely expressed in definitive erythroid lineages (c-myb, Sox6, and Bcl11A) in primitive erythroid cells from peripheral blood (PB) and in definitive proerythroblasts sorted from fetal liver (FL) and BM was determined by qPCR. Error bars indicate SEM and n = 3.
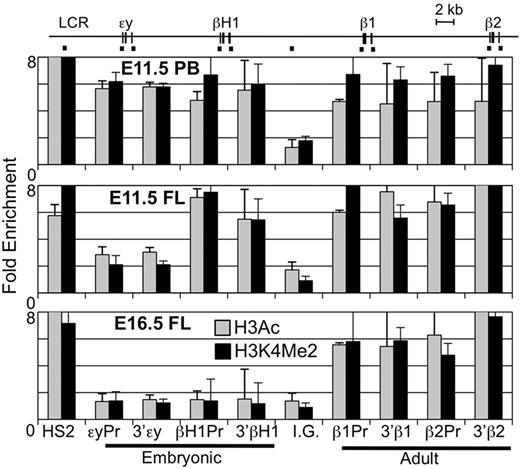
Various chromatin modifications have been associated with the lineage-specific expression of β-globin genes.22,35 Specific hyperacetylated and methylated forms of histones are found highly associated with transcribed β-globin genes during primitive, late fetal liver, and BM erythropoiesis. We compared the H3Ac and H3K4Me2 chromatin modifications across the β-globin locus using ChIP. In agreement with published data, primitive erythroid cells (E11.5 peripheral blood) have high levels of modified histones over both the embryonic (εy, βH1-) and adult (β1, β2) β-globin gene regions while cells in the E16.5 fetal liver had modified chromatin specifically associated with the adult β-globin genes (Figure 6).22 However, examination of the E11.5 liver revealed a previously undescribed pattern of highly modified histones associated both with the adult and the βH1-globin genes but not with εy-globin gene. These results are consistent with the unique expression and RNA Pol II binding of the βH1-, but not εy-globin, genes in EMP-derived erythropoiesis.
Unique chromatin modification patterns of the β-globin locus in primitive, EMP-derived fetal, and HSC-derived adult erythropoiesis. ChIP of modified histones (H3Ac or H3K4Me2) in the β-globin locus. Locations of amplimers are indicated by black bars in the diagram above and enrichments graphed in order below. Enrichment of H3Ac and H3K4Me2 was found in primitive erythroid cells from E11.5 peripheral blood (PB) in both embryonic and adult globin genes regions but only in adult genes in later fetal liver (E16.5 FL). E11.5 fetal liver had enrichment of modified histones over the adult genes and the βH1-globin gene but not εy-globin. Error bars represent the SEM and n = 3.
Unique chromatin modification patterns of the β-globin locus in primitive, EMP-derived fetal, and HSC-derived adult erythropoiesis. ChIP of modified histones (H3Ac or H3K4Me2) in the β-globin locus. Locations of amplimers are indicated by black bars in the diagram above and enrichments graphed in order below. Enrichment of H3Ac and H3K4Me2 was found in primitive erythroid cells from E11.5 peripheral blood (PB) in both embryonic and adult globin genes regions but only in adult genes in later fetal liver (E16.5 FL). E11.5 fetal liver had enrichment of modified histones over the adult genes and the βH1-globin gene but not εy-globin. Error bars represent the SEM and n = 3.
Early fetal definitive erythroid cells express high levels of human γ-globin in transgenic mice
Unlike most mammals, primates have different β-globin genes expressed in fetal versus adult erythroid cells. Transgenic mice with a single copy of the human β-globin locus have been widely used as a model to study the developmental regulation of the human β-globin genes.36,37 However, the results are difficult to interpret because of the coexistence of circulating primitive erythroid cells that express high levels of the “fetal” γ-globin transgene. To more precisely examine the expression of the human β-globin transgenes in each of the distinct erythroid lineages emerging during mammalian ontogeny, we performed qPCR of globin message in circulating primitive erythroid cells or sorted definitive proerythroblasts from early fetal (EMP-derived), late fetal and adult (HSC-derived) transgenic mice. Analysis of the endogenous murine β-globin genes confirmed the erythroid lineage identity and purity of these cells (Figure 7A). Primitive erythroid cells express both ε- and γ-globin transgenes, and ε-globin transcripts become more prominent as these cells mature in vivo. This mirrors the maturational globin switching observed in the endogenous murine genes, where the most abundant embryonic β-globin transcript is initially βH1- but then εy-globin is proportionally up-regulated as cells mature (Figure 7A).22 Both late fetal and adult proerythroblasts expressed predominantly the adult human β-globin transgene. These cells did not contain any murine εy- or βH1-globin message, and therefore were not contaminated with primitive erythroid cells. Nonetheless, we saw a small but significant expression of transgenic γ-globin gene in these late fetal liver proerythroblasts. This was not observed in the adult proerythroblasts indicating there is some underlying difference of globin gene regulation in the erythroid compartment of the fetal liver.
Human γ-globin is highly expressed in βH1-globin–expressing definitive proerythroblasts. (A) Mice with a single copy of the human β-globin locus18 were assayed by qPCR for gene expression of murine (top) and human (bottom) β-globin genes. Levels of globin transcripts are graphed as the percentage of total β-globin message measured from that locus. The left 3 samples are from primitive erythroid cells sorted from fetal blood (Ter119+ c-kit−). The middle 3 samples are from sorted definitive proerythroblasts (Ter119lo c-kit+) from fetal liver or BM. The right 2 samples are cultured E9.5 yolk sac EMP in erythroid maturation media. Error bars derived from the SEM of 3 independent experiments. (B) Three erythroid lineages emerge during mammalian embryogenesis. Two of these lineages, primitive erythroid and EMP-definitive erythroid, originate in the yolk sac. The AGM and other sites generate the HSC that colonize the liver and eventually the BM. Primitive erythroid precursors mature in the circulation, while EMP- and HSC-derived BFU-E mature in the liver of the fetus. Different patterns of β-globin expression in the mouse, human, and of the transgenic human genes in mice characterize these different forms of erythropoiesis (Figures 3, 7A).44,46-48 Genes are presented in their order within the β-globin locus. Grayed gene names signify no expression in that lineage. †While the yolk sac is the only reported site of EMP emergence precirculation, EMP may also be produced elsewhere and obscured from detection by the presence of EMP in circulating blood. *Maturational globin switching is observed in the first 2 lineages in mouse where βH1-globin or transgenic human γ-globin decrease in prevalence as erythroid precursors mature. ⋀β-globin is the major β-globin expressed in adult humans, however, rare γ-globin–expressing F cells are also found in the circulation.
Human γ-globin is highly expressed in βH1-globin–expressing definitive proerythroblasts. (A) Mice with a single copy of the human β-globin locus18 were assayed by qPCR for gene expression of murine (top) and human (bottom) β-globin genes. Levels of globin transcripts are graphed as the percentage of total β-globin message measured from that locus. The left 3 samples are from primitive erythroid cells sorted from fetal blood (Ter119+ c-kit−). The middle 3 samples are from sorted definitive proerythroblasts (Ter119lo c-kit+) from fetal liver or BM. The right 2 samples are cultured E9.5 yolk sac EMP in erythroid maturation media. Error bars derived from the SEM of 3 independent experiments. (B) Three erythroid lineages emerge during mammalian embryogenesis. Two of these lineages, primitive erythroid and EMP-definitive erythroid, originate in the yolk sac. The AGM and other sites generate the HSC that colonize the liver and eventually the BM. Primitive erythroid precursors mature in the circulation, while EMP- and HSC-derived BFU-E mature in the liver of the fetus. Different patterns of β-globin expression in the mouse, human, and of the transgenic human genes in mice characterize these different forms of erythropoiesis (Figures 3, 7A).44,46-48 Genes are presented in their order within the β-globin locus. Grayed gene names signify no expression in that lineage. †While the yolk sac is the only reported site of EMP emergence precirculation, EMP may also be produced elsewhere and obscured from detection by the presence of EMP in circulating blood. *Maturational globin switching is observed in the first 2 lineages in mouse where βH1-globin or transgenic human γ-globin decrease in prevalence as erythroid precursors mature. ⋀β-globin is the major β-globin expressed in adult humans, however, rare γ-globin–expressing F cells are also found in the circulation.
The EMP-derived definitive proerythroblasts in the early liver, unlike their HSC-derived definitive counterparts, express γ-globin as the predominant human transgene (Figure 7A). This suggests that the regulatory milieu of the β-globin locus in these cells may be closest to that of human fetal erythroblasts. The predominant expression of the human γ-globin transgene was also found in erythroblasts derived from E9.5 yolk sac BFU-E when cultured in vitro (Figure 7A). This finding once again links the unique globin expression in early liver definitive erythropoiesis to the EMP wave of hematopoiesis. Interestingly, maturational switching of human γ-to-β-globin genes, similar to that observed with murine βH1-to-β1-globin genes, occurs between days 2 and 3 of in vitro culture (Figure 7A). Indeed, in both yolk sac-derived lineages, there are qualitatively similar expression patterns of βH1- and human transgenic γ-globin genes suggesting the existence of shared regulation of these genes. Although we also note that within these patterns, γ-globin is always expressed at higher proportional levels among the human transgenes than βH1-globin is among the mouse globins.
Discussion
The terms “primitive” and “definitive” have historically been used to distinguish hematopoiesis during embryogenesis based on morphologic differences observed among the circulating erythroid cells.6,20 After the appearance of EryP-CFC, a second wave of hematopoietic potential, termed EMP, also emerges from the yolk sac.7,8,12,13 EMP-derived erythropoiesis is “definitive” based on its commonalities with adult erythropoiesis, including colony formation in semisolid media with BFU-E morphology and predominant expression of “adult” globins.8 In addition, the emergence of EMP-derived erythroid progenitors has been shown to require c-myb and Runx1, each of which are associated with “definitive” hematopoiesis.27,38-40 Considerable circumstantial evidence, including the expression of the definitive erythroid-specific Sox6 and Bcl11A transcription factors by erythroblasts in the liver before its colonization by HSC, further suggests that EMP-derived progenitors are the source of definitive erythropoiesis the early fetal liver (Figure 5). Furthermore, active erythropoiesis, consisting of all maturational stages of erythroid precursors from proerythroblasts to orthochromatic erythroblasts, is evident in the fetal liver at E11.5, when enucleated definitive erythrocytes are first found in the circulation, and when there is less than one HSC per liver in the mouse (Figure 1).3-5 Thus, another distinction between primitive and definitive erythropoiesis shared by both EMP- and HSC-derived erythropoiesis is that erythroid precursors mature in an extravascular microenvironment and are released into the bloodstream after their enucleation. These data indicate that there are 2 sources for definitive erythroid cells in the early embryo and that the first definitive erythrocytes are ultimately generated by EMP-derived hematopoietic progenitors (Figure 7B).
Our findings indicate that EMP-derived erythroid cells express a previously unreported pattern of β-globin genes (Figure 7B). Unlike primitive erythroblasts that express both “embryonic” βH1- and εy-globin genes, and adult erythroid cells that express neither, primary definitive erythroblasts in the early fetal liver uniquely express only the βH1-globin gene. In these cells, RNA polymerase is bound to the βH1-, but not the εy-globin genes, indicating that these genes are under differential transcriptional control. Increased histone hyperacetylation and H3K4 dimethylation present over the βH1-globin but not εy-globin genes in this lineage correlate with this unique expression pattern and exposes a heretofore unrecognized ability of murine erythroid cells to differentially modify the chromatin of its 2 “embryonic” genes. One model of murine β-globin gene regulation is based on inhibition of εy- and βH1-globin transcription in fetal and adult definitive erythropoiesis by expression of Bcl11A and Sox6, transcription factors not expressed in primitive erythroid cells.28,29,34,41 The presence of a definitive erythroid lineage that independently regulates the expression of βH1-globin and εy-globin genes in vivo, irrespective of the expression of Bcl11A and Sox6, requires a refinement of this model. While Sox6 transcript levels are significantly lower in E11.5 proerythroblasts than in other definitive proerythroblasts, the significance of this finding is unclear, because we still see εy-globin inhibition in this lineage which is linked to Sox6 function.28,42 Alternate splicing of Bcl11A to shorter forms has been reported and we used published primers designed to distinguish only longer transcripts34 but still found the same transcript levels in E11.5 liver and later proerythroblasts (data not shown). The unavailability of sufficient primary cells precluded Bcl11A protein analysis, so it is possible that Bcl11A function is regulated posttranscriptionally as has been proposed in primate erythropoiesis.29,34,43
In humans, primitive erythroid cells express predominantly ε-globin and a small amount of γ-globin.44 However, there is an additional switch in β-globin gene usage not found in nonprimate species, as fetal erythroid cells express predominantly γ-globins and adult erythroid cells express predominantly β-globin.41 Transgenic mice carrying the human β-globin locus have been used to model globin gene regulation.36,37 While there are reports of γ-globin expression in the fetal liver of these mice, it has recently been suggested that circulating primitive erythroid cells expressing γ-globin account for that expression.29 We used flow cytometry to purify single erythroid lineage cell populations from transgenic mice and confirmed their purity by appropriate coexpression of endogenous murine globin genes. As expected, primitive erythroid cells express both ε-globin and γ-globin transcripts. However, we also found expression of the human γ-globin transgene in late fetal liver proerythroblasts. Although much less abundant than adult β-globin messages, γ-globin transcripts are still detected in late fetal liver and not in transgenic BM proerythroblasts. These data agree with reports in transgenic mice showing that fetal livers have higher ratios of γ- to ε-globin transcripts and higher proportions of cells expressing γ- versus εy-globin than in fetal blood samples from the same embryos.29,45 In contrast to the low levels of transgenic γ-globin expressed in HSC-derived fetal liver cells, we found that EMP-derived definitive erythroid cells express predominantly γ-globin. Thus, the globin expression pattern in this transient lineage is most reminiscent of fetal human erythroid cells. While this similarity may suggest a model of prolonged EMP-derived definitive erythropoiesis in fetal humans, there is evidence that the ontogeny of erythropoiesis in the human closely resembles the mouse with overlapping yolk sac-derived and HSC-derived definitive erythropoiesis. At 4 to 5 weeks in human embryos, similar to E9.5 to E10.5 in mouse, circulating erythroid cells are primitive, while the first definitive BFU-E are found in the yolk sac and subsequently transition to the liver.46,47 These first BFU-E obtained from human embryos also express predominantly γ-globin when cultured in vitro.48 At this same time, HSC formation is proposed to begin as clusters of budding cells in the dorsal aorta.49 These results support the concept that primitive, EMP-derived definitive, and HSC-derived definitive erythroid lineages are conserved between mouse and human (Figure 7B).
In summary, our understanding of hematopoietic ontogeny in the mammalian embryo must be expanded to encompass 3 distinct erythroid lineages, each with a unique pattern of β-globin gene expression. These 3 lineages provide the constantly increasing numbers of erythroid cells needed by the rapidly growing embryo. Primitive erythropoiesis generates circulating erythroblasts that expand 100-fold between E8.5 and E10.5 of mouse embryogenesis.50 However, the expansion of primitive erythroid cells drastically decreases after E10.5 as these cells complete their terminal stages of maturation.1,50 HSC have just begun to colonize the fetal liver by E11.5-E12.5 and subsequently expand in numbers.4 We hypothesize that EMP-derived definitive erythropoiesis, ultimately originating within the yolk sac, fills the gap between the terminal maturation of the primitive erythroblasts and establishment of HSC erythropoiesis. The complexity of erythropoiesis in the mammalian embryo creates significant challenges when interpreting experiments in the embryo as well as surrogate embryonic systems such as ES cells and iPS cells cultured in vitro. However, it also affords us the opportunity to gain unique insights into the onset and regulation of erythropoiesis through comparative analysis of these lineages.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The gift of the transgenic mice containing the human β-globin locus from Keiji Tanimoto is gratefully acknowledged. Expert technical assistance was provided by Shay Karkashon.
This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK09361; J.P.) and the Michael Napoleone Memorial Foundation. Generous financial support came from the Albert Einstein College of Medicine (J.L.).
National Institutes of Health
Authorship
Contribution: K.E.M. designed and performed experiments, analyzed data, and wrote the manuscript; J.M.F., G.J.F., A.D.K, and P.D.K. designed and performed experiments and analyzed data; J.L. designed experiments and provided time pregnant transgenic mice; M.B. designed experiments; and J.P. designed and performed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for G.J.F. is National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC.
Correspondence: Dr James Palis, University of Rochester Medical Center, 601 Elmwood Ave, Box 703, Rochester, NY 14642; e-mail: james_palis@urmc.rochester.edu.