Abstract
Under persistent antigenic stimulation, virus-specific CD8+ T cells become increasingly dysfunctional and up-regulate several inhibitory molecules such as killer lectin-like receptor G1 (KLRG1). Here, we demonstrate that HIV-1 antigen-specific T cells from subjects with chronic-progressive HIV-1 infection have significantly elevated KLRG1 expression (P < .001); show abnormal distribution of E-cadherin, the natural ligand of KLRG1, in the intestinal mucosa; and have elevated levels of systemic soluble E-cadherin (sE-cadherin) that significantly correlate with HIV-1 viral load (R = 0.7, P = .004). We furthermore demonstrate that in the presence of sE-cadherin, KLRG1hi HIV-1–specific CD8+ T cells are impaired in their ability to respond by cytokine secretion on antigenic stimulation (P = .002) and to inhibit viral replication (P = .03) in vitro. Thus, these data suggest a critical mechanism by which the disruption of the intestinal epithelium associated with HIV-1 leads to increased systemic levels of sE-cadherin, which inhibits the effector functions of KLRG1hi-expressing HIV-1–specific CD8+ T cells systemically.
Introduction
Cytolytic CD8+ T-cell responses have been shown to play a major role in the pathogenesis of viral infections. After acute viral infection, virus-specific CD8+ T cells undergo sequential activation and expansion, and in the process they acquire effector functions such as the production of the antiviral cytokines IFN-γ and TNF-α.1 However, during persistent viral infections the function and differentiation of virus-specific CD8+ T cells become progressively “exhausted” as a consequence of persistent viremia.2-4 Several molecules have been identified as important mediators of T-cell exhaustion, including programmed death-1 (PD-1),3,5 CTLA-4,4 and Lag3.6 It is thought that each of these molecules serves to regulate the function of antigen-specific CD8+ T cells, yet the measure of regulation and interdependence of each of these pathways is unknown.
The killer cell lectin-like receptor G1 (KLRG1) plays a unique but poorly characterized role in mediating T-cell exhaustion. KLRG1 is expressed on a subset of CD4+, CD8+ T cells, as well as on natural killer cells.7-9 On CD8+ T cells, KLRG1 is upregulated on virus-specific T cells in response to repetitive antigenic stimulation. Indeed, most virus-specific CD8+ T cells are KLRG1+ in chronic viral infections such as CMV and EBV, but not in cleared infections such as influenza.10-12 However, the role of KLRG1 in HIV-1 infection remains largely unknown.12
The ligand for KLRG1 was identified as E-cadherin, a member of the cadherin (calcium-dependent adhesion molecules) family of type I transmembrane proteins that forms tight intracellular connections between cells within epithelial surfaces.13-15 It is expressed at high levels within the gastrointestinal epithelium, and disruption of the integrity of mucosal membranes that occurs in the setting of invasive gastrointestinal cancers or chronic inflammatory bowel disease can lead to the release of a soluble form of E-cadherin (sE-cadherin) into the plasma.16-19 Although elevated intestinal mucosal permeability has been described to play a critical role in HIV-1 pathogenesis,20,21 changes in soluble E-cadherin levels have not been investigated.
One of the most striking characteristics of HIV-1 infection is the profound pathologic changes that occur in the gastrointestinal tract. Enteropathic changes such as diarrhea, malabsorption, and weight loss are hallmarks of HIV-1 infection and have been associated with a profound intestinal depletion of CD4+ T cells.22-24 This depletion occurs within the first 10-14 days of infection, introducing structural abnormalities such as the loss of gastrointestinal mucosal epithelial integrity, greater mucosal permeability, and increased translocation of luminal bacterial products, which result in elevated plasma bacterial lipopolysaccharide (LPS). Plasma LPS levels correlate with peripheral T-cell activation and have been suggested to play a key role in the immunopathogenesis of HIV-1.21,25 After the acute phase of infection, HIV-1–specific CD8+ T-cell responses are detectable in the gastrointestinal tract; however, the containment of viral replication in the gastrointestinal mucosa appears to be impaired.26-28
Here, we present a novel mechanism of inhibition of important antiviral functions of HIV-1–specific CD8+ T cells that links pathologic changes in the gut with systemic immune dysfunction. We report that HIV-1–specific CD8+ T cells significantly up-regulate KLRG1 in chronic HIV-1 infection. We also show a disruption of the normal distribution of colonic E-cadherin in patients with HIV-1 infection coincident with increases of sE-cadherin in the plasma. The presence of sE-cadherin in turn impairs the ability of HIV-1–specific CD8+ T cells to exert antiviral functions and might contribute to a vicious cycle of low viral containment, elevated levels of antigenemia, and increased CD8+ T-cell dysfunction.
Methods
Study subjects
Subjects positive or negative for HIV-1 were recruited at the Massachusetts General Hospital after giving informed consent. Subjects with HIV-1 chronic-progressive disease were defined as being infected for ≥ 1 year with stable viral loads > 10 000 copies/mL. Subjects with controlled HIV-1 infection (termed elite controllers) were defined as HIV-1+ for ≥ 1 year with stable viral loads below the limit of detection (< 50 copies/mL) in the absence of antiretroviral therapy (ART). Subjects on ART had a fully suppressed viral load (< 50 copies/mL) and were on treatment for ≥ 6 months (Table 1). The study was approved by the respective institutional review boards and was conducted in accordance with human experimentation guidelines of the Massachusetts General Hospital.
KLRG1 expression on HIV-1–specific CD8+ T cells
Cryopreserved PBMCs were thawed, resuspended to 1-2 × 106 cells/mL in R10 media (RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, 5.5 mL of HEPES buffer; pH 7.2 ± 0.2), and rested for 1-2 hours at 37°C, 5% CO2. Cells were then washed with PBS and stained for intracellular amine groups to discriminate between live and dead cells (violet viability dye; Invitrogen). After an additional wash with 2% FCS/PBS, allophycocyanin (APC)–labeled HLA class I tetramers or pentamers (Beckman-Coulter, NIH, Proimmune) refolded with the respective CD8+ T-cell epitopes were added. After 20 minutes of incubation at room temperature, cells were stained with the surface antibodies anti–CD3-phycoerythrin-Cy5.5 (PeCy5.5; Invitrogen), anti–CD8-APC-Cy7 (BD Biosciences), and anti–PD-1-FITC (eBiosciences). Anti–CD14-PacificBlue (eBiosciences) and anti–CD19-PacificBlue (eBiosciences) were added to exclude monocytes and B cells. After an additional wash step cells were fixed with 1% paraformaldehyde, stained with anti-KLRG1 (Santa Cruz Biotechnology), washed, and stained with anti–rabbit Pacific Orange (Invitrogen). Expression of KLRG1 on HIV-1–specific cells before and after sequence variations was assessed on CD8+ T-cell responses we have previously published. All data were collected on a BD LSRII (BD Biosciences) flow cytometer and analyzed with FlowJo 8.3.3 software (TreeStar). Initial gating was on the lymphocyte population, and then a forward scatter width versus forward scatter height plot was used to remove doublets.
Measurement of sE-cadherin, sCD14, and LPS in plasma and E-cadherin tissue levels by ELISA
sE-cadherin plasma levels were measured by quantitative ELISA (R&D Systems) according to the manufacture's protocol. In brief, a monoclonal antibody specific for E-cadherin was precoated onto a microplate. Standards and samples were pipetted into the wells, and any E-cadherin present was bound by the immobilized antibody. After washing away any unbound material, an enzyme-linked polyclonal antibody specific for E-cadherin was added to the wells. After a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells, and color was developed in proportion to the amount of E-cadherin bound in the initial step. The color development was stopped, and the intensity of the color was measured. Each plasma sample was measured in duplicate. The concentration levels were read by reducing the data to a 4-parameter logistic curve-fit as suggested. The same ELISA was used to detect E-cadherin levels in tissue. For this, snap-frozen colonic tissue samples were weighed and homogenized in PBS with 0.1% Tween with the use of a roto-stator for 60 seconds. The homogenized tissue was then spun in a microcentrifuge at 10 000 rpm for 15 minutes to remove debris. The supernatants were then used in the E-cadherin ELISA protocol as described earlier. LPS (Limulus Amebocyte assay; Cambrex) and sCD14 levels (Quantikine; R&D Systems) were measured on the same plasma samples according to the manufacture's protocol and were previously described.20
Immunohistochemistry staining for E-cadherin in the gastrointestinal tract
A database of formalin-fixed, paraffin-embedded pathologic samples was searched for pinch biopsies taken from the colon of HIV-1–negative and chronically HIV-1–infected subjects. These specimens were obtained during the course of medical care at the Brigham and Women's Hospital. Samples were deidentified but were accompanied by CD4 count, HIV-1 viral load, and treatment status in accordance with a protocol approved the institutional review board. Sections (4 μm) were made, and antigen retrieval was performed by placing slides in EDTA pH 8.0 (Invitrogen) and treating them in a pressure cooker. The slides were then blocked with peroxidase block (Dako) and stained with a mouse anti–human E-cadherin primary antibody (Clone 36; BD Bioscience) diluted 1:100 in antibody diluent (Dako). After incubation for 1 hour at room temperature, the slides were washed with 50mM Tris and stained with a goat anti–mouse antibody conjugated to the enzyme HRP (Dako). Slides were again washed, and antibody staining was visualized with the addition of a chromogenic substrate, DAB (3–3′-diaminobenzidine; Dako). Slides were then washed and counterstained with hematoxylin (Sigma-Aldrich). Images were recorded on a Nikon inverted microscope equipped with a color CCD camera (20×/0.75 NA Plan APO; Aperio Imagescope viewer). Quantitation of E-cadherin staining was performed by selecting 5 representative regions of the lamina propria from each section. HIV status of the patients was concealed during the analysis. The percentage of staining within these regions of the lamina propria was then quantitated with image analysis software (Aperio Inc), and averages were calculated for each specimen.
Assessment of the functional profile of KLRG1+ or KLRG1− T cells and antigen-specific T-cell function in the presence or absence of sE-cadherin by multiparameter flow cytometry
Cryopreserved PBMCs were thawed and PBMCs were examined for viability by Trypan blue exclusion (typically 80%-90% viable) and adjusted to 1 × 106 cells/mL. Costimulatory antibodies (CD28 and CD49d, 1 μg/mL; BD Biosciences) and CD107a-PECy5 (BD Biosciences) were added, and the cells were divided into aliquots at 1 mL to each tube containing 2 μg/mL Gag peptide pool. An unstimulated (R10 only) and a positive control (staphylococcal enterotoxin B) were included in each assay. sE-cadherin was added at a final concentration of 1 μg/mL (the optimal sE-cadherin concentration was determined by serial dilution). Cells were incubated for a total of 6 hours at 37°C, 5% CO2 in the presence of monensin (Golgistop; 0.7 μL/mL; BD Biosciences) and brefeldin A (10 μg/mL; Sigma-Aldrich). Cells were then washed with PBS and stained for intracellular amine groups to discriminate between live and dead cells (violet viability dye; Invitrogen). After an additional wash the following surface markers were added: anti–CD3-PeCy5.5 (Invitrogen), anti–CD8-APC-Cy7 (BD Biosciences), anti–CD19-Pacific Blue (eBiosciences). After a wash cells were fixed with 1% paraformaldehyde, stained for anti-KLRG1 (Santa Cruz Biotechnology), washed, and stained with anti–rabbit Pacific Orange (Invitrogen). Cells were then permeabilized (Fix Perm B solution; Caltag Laboratories) and stained intracellularly with the use of a panel of IL-2–FITC (BD Biosciences), IFN-γ–PE-Cy7 (BD Biosciences), and TNF-α–Alexa 700 (BD Biosciences). Between 150 000 and 500 000 events were collected per sample on a BD LSRII multicolor flow cytometer. Initial gating was on the lymphocyte population and then a forward scatter width versus forward scatter height plot was used to remove doublets. Subsequently, the events were gated through a side scatter versus violet viability dye (Pacific Blue) and sequentially gated on CD3+ and CD8+ events. After identification of CD8+ T cells, a gate was made for each respective function with the use of combinations that provided optimal separation. After the gates for each function were created, we used the Boolean gate platform to create the full array of possible combinations, equating to 16 response patterns when testing 4 functions. Data are reported after background correction, and the percentage of epitope-specific CD8+ T-cell responses had to be ≥ 2-fold higher than background for individual cytokines or CD107a before using the Boolean gate platform to be considered as a positive response.
Immunofluorescence staining for E-cadherin
Immunofluorescence staining was performed with the use of 4-μm thick formalin-fixed, paraffin-embedded tissue sections. Briefly, slides were deparaffinized in xylene and then passed through graded alcohols and put in distilled water. To reduce autofluorescence, tissue was treated with 1% Sudan Black in 70% EtOH. Antigen retrieval was performed in a steam pressure Decloaking Chamber (BioCare Medical) with 10mM citrate, pH 6.0 (Invitrogen). Slides were washed in 50mM Tris-HCl, pH 7.4 in between all further steps. Pretreatment included Peroxidase Block (Dako) followed by incubation with Protein Block, Serum Free (Dako). Monoclonal mouse anti–E-Cadherin (1:200 dilution; clone 36/E-Cadherin; BD Bioscience) diluted in Dako Diluent was applied for 1 hour followed by anti–mouse Envision (Dako) secondary antibody for 1 hour. After washing, Cy5-tyramide Signal Amplification System (Perkin-Elmer Life Science Products) was applied as per manufacturer's instructions. Costaining was performed sequentially by incubating for 1 hour with anti–human CD19 (1:2000 dilution; clone LE-CD19; AbD Serotec), anti–human CD68 (1:200 dilution; clone PG-M1; Dako), or polyclonal rabbit anti–human CD3(1:250 dilution; Dako). Coverslips were mounted onto slides with Prolong Gold Anti-fade Reagent with DAPI (4′-6′-diamidine-2-phenylindole; Molecular Probes) to visualize all nuclei. Images were acquired with a Zeiss LSM510 laser scanning confocal microscope
Luminex assay
Cryopreserved PBMCs were thawed and cells (1 × 106) were stimulated in a 24-well plate with Gag peptide pool (final concentration, 2 μg/mL). Recombinant sE-cadherin was added at a concentration of 1 μg/mL. Supernatant fluid was collected after 8 hours of stimulation. The cytokine levels of IFN-γ, TNF-α, and IP-10 or IFN-γ–inducible protein 10 were assessed with the use of a standard Luminex multiplexed bead system (Milliplex) according to the manufacturer's instructions. Results obtained from the Bio-Plex system were analyzed automatically by the Bio-Plex Manager software program (Bio-Rad Laboratories Inc) with a (5-parameter logistic fit) standard curve derived from recombinant cytokine and chemokine standards.
Akt(Ser473) Phosflow assay
Phosphorylation of Akt(Ser473) was assessed as previously described.29 In brief, cryopreserved PBMCs were thawed and rested overnight. Cells were then stimulated with anti-CD3 antibody (clone CD3-2; Mabtech) cross-linked with goat anti–mouse Ig (BD Biosciences) for 1 hour in the presence or absence of sE-cadherin in a water bath at 37°C. Cells were immediately fixed with BD Cytofix and permeabilized with Phosflow III solution (BD Biosciences). Cells were consecutively stained for CD3, CD4, CD8, and Akt(Ser473) and acquired on a 4-laser BD LSRII.
Viral inhibition assays
Autologous CD4+ lymphocytes were stimulated with a CD3/8 bispecific antibody and infected at day 3 with NL4-3 HIV-1 isolate at a MOI 0.01 for 4 hours at 37°C, washed twice, resuspended in medium, and plated at 105 cells/well. Effector cells, consisting of purified CD8+ T cells from the same donor, were separated, rested in medium alone, and added at an effector/target concentration of 1:1 in the presence or absence of recombinant E-cadherin. KLRG1 expression levels of the same cells were measured at the same time. A control with infected autologous CD4+ T cells alone and in uninfected CD4+ T cells served as positive and negative control. Supernatant fluid was collected at baseline and on days 3, 5, and 7. Viral replication was quantified by p24 Elisa (Perkin-Elmer) in duplicates.
Statistical analysis
Unless otherwise noted, all data were background-subtracted, and a lower threshold corresponding to at least twice above background was built for individual cytokine, chemokine, and CD107a expression in the flow cytometric-based experiments. Values below this threshold were set to 0. PD-1 expression was assessed by median fluorescence intensity and compared with a Fluorescent Minus One control. Statistical analyses were performed with the use of GraphPad Prism 4.0 and Excel (Microsoft Office). Two groups were compared with a Mann-Whitney test, whereas T-cell functions in the presence or absence of sE-cadherin were assessed by Wilcoxon matched pairs test unless otherwise noted. Correlations were based on a Spearman rank test. A P value < .05 was considered statistically significant.
Results
Elevated KLRG1 expression on HIV-1–specific CD8+ T cells from persons with progressive infection
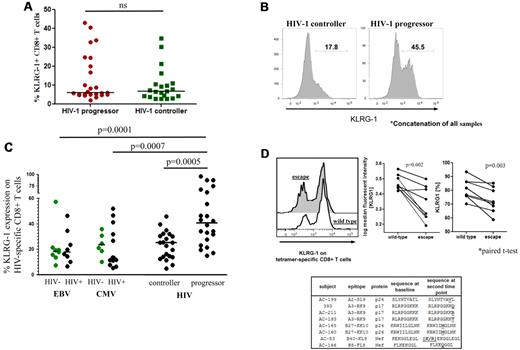
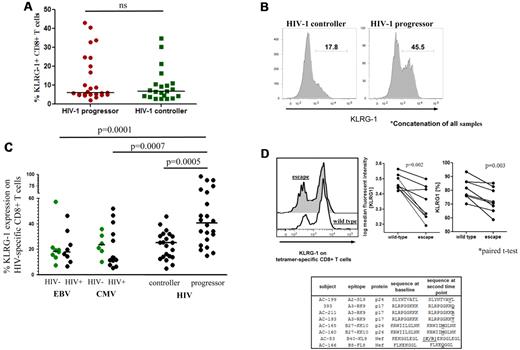
Previous studies have reported an increase of KLRG1 expression on virus-specific CD8+ T cells in persistent viral infections such as hepatitis C virus, EBV, and CMV.11,12 However, little is known about the expression of KLRG1 in chronic HIV-1 disease.12 We therefore examined the expression level of KLRG1 on CD8+ T cells from untreated subjects with chronic-progressive HIV-1 infection (“HIV-1 progressors,” average viral load > 10 000 RNA copies/mL in the absence of ART) and in persons with controlled HIV-1 infection (“HIV-1 elite controllers,” average viral load < 50 RNA copies/mL in the absence of ART). KLRG1 expression was variable on CD8+ T cells in HIV-1 infection and showed no statistically significant difference in expression levels between the 2 groups on bulk CD8+ T cells (Figure 1A; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). KLRG1 expression on HIV-1–specific CD8+ T cells identified with MHC class I tetramers, however, was up-regulated on a greater percentage of antigen-specific cells and at significantly higher levels in HIV-1 progressors than in HIV-1 controllers (P = .0005; Figure 1B-C). These expression levels were significantly higher compared with CMV- and EBV-specific CD8+ T-cell responses in both HIV-1–infected and –uninfected persons, showing that KLRG1 is specifically up-regulated on HIV-1–specific T cells (P = .0007 and P = .0001, respectively; Figure 1C). Further definition of the properties of KLRG1-expressing antigen-specific CD8+ T cells showed a predominantly CD127low, CCR7low, and CD45RAlow/positive phenotype, consistent with an effector/effector memory T-cell subset (supplemental Figure 2), in line with previous findings.12 KLRG1 expression did not correlate with the expression level of 2 other well-characterized inhibitory receptors: PD-1 and CTLA-4 (supplemental Figure 3A-C), but it was significantly up-regulated on repetitive stimulation in in vitro culture (supplemental Figure 3D). This suggests a distinct role of KLRG1 from these other immunoregulatory pathways, which has been previously noted in the lymphochoriomeningitis mouse model.6 However, the expression of KLRG1 did weakly correlate with levels of lymphocyte activation gene-3, a regulator of T-cell activation,30 and strongly with CD57, a marker for replicatively senescent terminal effector cells31,32 (supplemental Figure 3E). A previous study suggested that the combination of CD57 and KLRG1 markers may differentiate between memory (CD57−KLRG1+) and effector (CD57+KLRG1+) T-cell phenotypes,12 suggesting an accumulation of effector CD8+ T-cell populations in chronic-progressive HIV-1 infection.
Expression of KLRG-1 on HIV-1–specific CD8+ T cells. (A) The expression levels of KLRG-1 on bulk CD8+ T cells varies, but it is not significantly different between chronic progressors and elite controllers. The horizontal lines at each variable indicate the mean. (B) A concatenation of samples from 14 HIV-1 chronic progressors and 11 HIV-1 elite controllers assessed for KLRG1 expression levels on tetramer-positive HIV-1–specific CD8+ T cells by flow cytometry. (C) Median fluorescent intensity of KLRG1 expression on HIV-1–specific tetramer-positive cells from 14 HIV-1 chronic progressors and 11 HIV-1 elite controllers. EBV- and CMV-specific cells were also analyzed for KLRG1 expression in both HIV-infected and -uninfected subjects. Antigen-specific CD8+ T cells from subjects with chronic-progressive disease had significantly higher levels of KLRG1 expression than subjects with controlled disease or cells specific for CMV or EBV, both mediators of latent viral infections. The horizontal lines at each variable indicate the mean. (D) The reduction in KLRG1 expression on epitope-specific CD8+ T cells after sequence evolution in the respective epitopes.33 Primary flow data (left) and combined data for 8 subjects (right) for KLRG1 expression on epitope-specific CD8+ T cells before (wild-type) and after (escape) the selection of amino acid substitutions within the targeted epitopes are shown. The decrease of the KLRG-1 expression on tetramer-positive CD8+ T cells before and after the development of sequence variations within epitopes was statistically significant in frequency (P = .003 paired t test) and median fluorescent intensity (P = .002 paired t test).
Expression of KLRG-1 on HIV-1–specific CD8+ T cells. (A) The expression levels of KLRG-1 on bulk CD8+ T cells varies, but it is not significantly different between chronic progressors and elite controllers. The horizontal lines at each variable indicate the mean. (B) A concatenation of samples from 14 HIV-1 chronic progressors and 11 HIV-1 elite controllers assessed for KLRG1 expression levels on tetramer-positive HIV-1–specific CD8+ T cells by flow cytometry. (C) Median fluorescent intensity of KLRG1 expression on HIV-1–specific tetramer-positive cells from 14 HIV-1 chronic progressors and 11 HIV-1 elite controllers. EBV- and CMV-specific cells were also analyzed for KLRG1 expression in both HIV-infected and -uninfected subjects. Antigen-specific CD8+ T cells from subjects with chronic-progressive disease had significantly higher levels of KLRG1 expression than subjects with controlled disease or cells specific for CMV or EBV, both mediators of latent viral infections. The horizontal lines at each variable indicate the mean. (D) The reduction in KLRG1 expression on epitope-specific CD8+ T cells after sequence evolution in the respective epitopes.33 Primary flow data (left) and combined data for 8 subjects (right) for KLRG1 expression on epitope-specific CD8+ T cells before (wild-type) and after (escape) the selection of amino acid substitutions within the targeted epitopes are shown. The decrease of the KLRG-1 expression on tetramer-positive CD8+ T cells before and after the development of sequence variations within epitopes was statistically significant in frequency (P = .003 paired t test) and median fluorescent intensity (P = .002 paired t test).
Given the high expression levels of KLRG1 on activated HIV-1–specific CD8+ effector T cells, we hypothesized that escape mutations in a single epitope and consequential loss of epitope-specific stimulation of CD8+ T cells would result in decreased expression of KLRG1. The loss of a single antigen because of escape mutation in an HIV-1–infected person decreases the expression of PD-1 on the cell surface of cognate antigen-specific CD8+ T cells.33 Longitudinal samples of the previously studied subjects were used to investigate the expression of KLRG1 on antigen-specific CD8+ T cells with responses directed against 8 HIV-1 epitopes. In all samples the wild-type viral sequence was present at the first time point, but a CTL escape mutation had developed at the second time point that resulted in reduced recognition of the epitope.33 Similar to the expression of PD-1, KLRG1 expression levels significantly decreased on the 8 epitope-specific CD8+ T cells after the development of sequence variations in the targeted epitopes measured by frequency (81% ± 7.5% to 73% ± 9%; P = .003 paired t test; Figure 1D) or median fluorescence intensity (3009 ± 360 to 2251 ± 557; P = .002 paired t test; Figure 1D). Thus, the reduction of KLRG1 expression levels on epitope-specific CD8+ T cells after CTL escape further suggests that KLRG1 expression is regulated by antigenic stimulation.
Reduced polyfunctionality of KLRG1-expressing HIV-1–specific CD8+ T cells
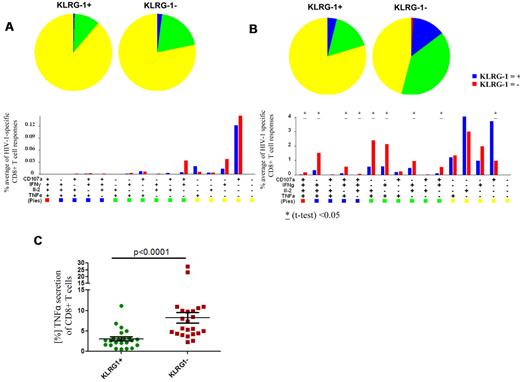
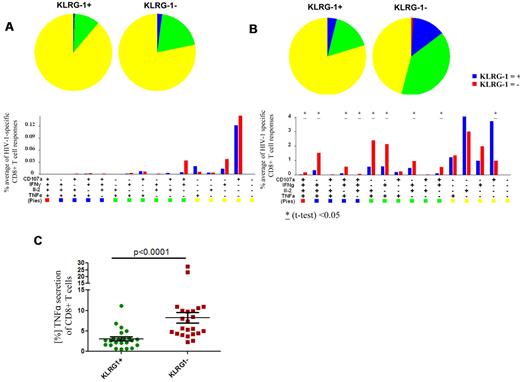
Virus-specific CD8+ T cells from subjects with chronic-progressive HIV-1 infection have an impairment in their ability to simultaneously secrete multiple cytokines (“polyfunctionality”) compared with HIV-1 elite controllers, and this has been reported to be associated with an exhausted T-cell phenotype.33,34 In the analysis of multifunctional T cells, 4 different functions (IFN-γ, IL-2, TNF-α, and CD107a) were investigated. We assessed whether the expression of KLRG1 correlated with polyfunctionality in 20 subjects with chronic HIV-1 infection. PMBCs were stimulated with appropriate HLA-matched optimal CD8+ T-cell epitopes selected on the basis of CD8+ T-cell responses previously characterized with the use of an IFN-γ ELISPOT assay.35 In subjects with chronic HIV-1 infection, most of the HIV-1–specific CD8+ T-cell responses were monofunctional to trifunctional.33,34 However, we noted that KLRG1+ CD8+ T-cell responses were, in comparison to KLRG1− CD8+ T-cell responses, less polyfunctional (Figure 2A). Interestingly, although TNF-α responses were generally low, no TNF-α responses in the HIV-1–specific CD8+ T cells expressing KLRG1 were present. Because TNF-α has been previously associated with the ability of HIV-1–specific CD8+ T cells to inhibit viral replication,36 we tested whether KLRG1+ cells are generally impaired in their ability to secrete TNF-α. We stimulated PBMCs of the same 20 subjects with phorbol 12-myristate 13-acetate and ionomycin (Figure 2B). Consistent with our previous finding, KLRG1+ cells were less capable of mounting a polyfunctional response and were primarily monofunctional. Moreover, most CD8+ T cells able to secrete TNF-α were KLRG1−, suggesting that KLRG1+ CD8+ T cells have a distinct functional role in the antiviral response compared with KLRG1− cells (P < .0001) (Figure 2C).
Multiparameter flow analysis of the polyfunctional profile of KLRG-1+ and KLRG-1− HIV-1–specific CD8+ T cells. Epitope-specific CD8+ T-cell responses of 20 subjects with chronic-progressive or controlled HIV-1 infection directed against 23 HIV-1 epitopes were tested (A3-RK9, B8-EI8, B8-FL8, B27-KK10, B57TW10, B57-KF11, B7-GL9). All 16 possible combinations of the 4 antigen-specific functions studied for each epitope are shown on the x-axis, and the contribution of each epitope-specific CD8+ T-cell population exhibiting the respective combination of functions is indicated as bars (see bar graphs on lower portion of figure). Responses are grouped and color-coded according to the number of functions (1 = yellow, 2 = green, 3 = blue, 4 = red). Blue bars show the results from the KLRG-1+ CD8+ T-cell responses, and red bars show the results from the KLRG1− responses. The data are summarized by pie charts in which each slice of the pie represents the fraction of the total epitope-specific response. Pie charts on the left show the responses of the KLRG1+ CD8+ T cells, and pie charts on the right show the KLRG1− fraction. (A) The optimal epitope HIV-1–specific CD8+ T-cell responses were predominantly monofunctional. Although the KLRG1− CD8+ T-cell responses appeared to contain a larger fraction of dual-functional and trifunctional responses, these differences were not statistically significant. To tease out differences in the responsive ability of KLRG1+ versus KLRG1− CD8+ T cells, we stimulated PBMCs of the same 20 subjects with phorbol 12-myristate 13-acetate and ionomycin (B). The ability to respond to this stimulus between KLRG1+ and KLRG1− cells was significantly different. Although KLRG1+ CD8+ T cells responded predominantly with monofunctional responses, we observed significantly more polyfunctional responses from KLRG1− CD8+ T cells. Significant differences are indicated; *P < .05. We also noted that most TNF-α secretion was from KLRG1− CD8+ T cells, whereas relatively small amounts of TNF-α were produced by KLRG1+ CD8+ T cells (P < .0001). The horizontal lines at each variable indicate the mean (C).
Multiparameter flow analysis of the polyfunctional profile of KLRG-1+ and KLRG-1− HIV-1–specific CD8+ T cells. Epitope-specific CD8+ T-cell responses of 20 subjects with chronic-progressive or controlled HIV-1 infection directed against 23 HIV-1 epitopes were tested (A3-RK9, B8-EI8, B8-FL8, B27-KK10, B57TW10, B57-KF11, B7-GL9). All 16 possible combinations of the 4 antigen-specific functions studied for each epitope are shown on the x-axis, and the contribution of each epitope-specific CD8+ T-cell population exhibiting the respective combination of functions is indicated as bars (see bar graphs on lower portion of figure). Responses are grouped and color-coded according to the number of functions (1 = yellow, 2 = green, 3 = blue, 4 = red). Blue bars show the results from the KLRG-1+ CD8+ T-cell responses, and red bars show the results from the KLRG1− responses. The data are summarized by pie charts in which each slice of the pie represents the fraction of the total epitope-specific response. Pie charts on the left show the responses of the KLRG1+ CD8+ T cells, and pie charts on the right show the KLRG1− fraction. (A) The optimal epitope HIV-1–specific CD8+ T-cell responses were predominantly monofunctional. Although the KLRG1− CD8+ T-cell responses appeared to contain a larger fraction of dual-functional and trifunctional responses, these differences were not statistically significant. To tease out differences in the responsive ability of KLRG1+ versus KLRG1− CD8+ T cells, we stimulated PBMCs of the same 20 subjects with phorbol 12-myristate 13-acetate and ionomycin (B). The ability to respond to this stimulus between KLRG1+ and KLRG1− cells was significantly different. Although KLRG1+ CD8+ T cells responded predominantly with monofunctional responses, we observed significantly more polyfunctional responses from KLRG1− CD8+ T cells. Significant differences are indicated; *P < .05. We also noted that most TNF-α secretion was from KLRG1− CD8+ T cells, whereas relatively small amounts of TNF-α were produced by KLRG1+ CD8+ T cells (P < .0001). The horizontal lines at each variable indicate the mean (C).
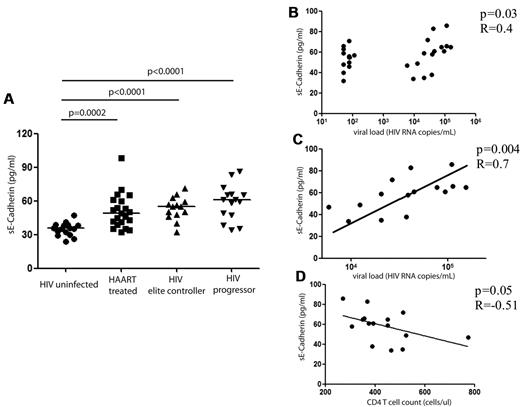
sE-cadherin levels are elevated in HIV-1 infection
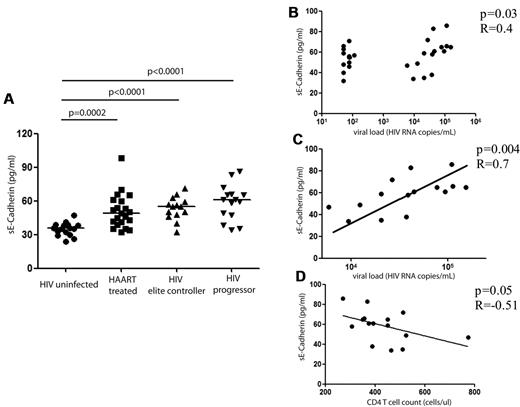
To further investigate the biologic consequences of KLRG1 up-regulation on CD8+ T cells in HIV-1 infection, we determined the levels of the natural ligand for KLRG1, E-cadherin, in subjects in different stages of HIV-1 infection. E-cadherin is an adhesion molecule that plays an important role in maintaining the integrity of epithelial surfaces,37 and measurable levels in the plasma are indicative of the disruption of epithelial membranes.38 E-cadherin is expressed at high levels in the epithelial tissue within the gastrointestinal tract,39,40 and the magnitude of plasma levels of sE-cadherin has been used as markers of disease recurrence and predictors of outcome in invasive gastrointestinal carcinoma.41 We therefore analyzed sE-cadherin levels cross-sectionally in the plasma of subjects with chronic-progressive HIV-1 infection, spontaneously controlled HIV-1 infection, HIV-1+ subjects on ART for > 6 months, and HIV-negative subjects. Significantly higher sE-cadherin levels were detected in all persons infected with HIV-1 (P < .0001 to P = .0002) (Figure 3A). This was the case for subjects with controlled viral replication off ART as well as for subjects on ART or with chronic untreated HIV-1 infection. We noted indications of lower sE-cadherin levels in subjects with controlled HIV-1 infection (either spontaneous control or control with ART); however, these differences were not statistically significant (P = .05). In untreated HIV-1–infected persons with sustained plasma viremia, HIV-1 viral load was significantly correlated to sE-cadherin plasma levels (P = .03, R = 0.4; Figure 3B). Interestingly, we observed a broad spectrum of sE-cadherin levels in HIV controllers, suggesting differences in the level of gut leakage in this subset of patients. However, we observed a strong correlation to sE-cadherin plasma levels in chronic progressors alone (P = .004, R = 0.7; Figure 3C), whereas sE-cadherin plasma levels were not correlated to levels of LPS or soluble CD14 in the plasma (supplemental Figure 4), both factors that have been reported to be elevated in subjects with increased mucosal permeability.20 In addition, the CD4+ T-cell count in subjects with chronic HIV-1 infection was inversely correlated with the plasma level of sE-cadherin (Figure 3D; P = .05, R = −0.51).
sE-cadherin levels in different stages of HIV-1 infection. The levels of sE-cadherin in the plasma were assessed by ELISA and are reported (in pg/mL). sE-cadherin levels were compared in HIV-negative subjects (n = 16), HIV-positive subjects on ART (n = 21), elite controllers (n = 14), and subjects with chronic-progressive HIV-1 infection (“progressors”; n = 15). sE-cadherin levels were significantly higher in all HIV-1 infected subjects compared with HIV-1 negative subjects (ART, P = .0002; elite controllers and progressors, P < .0001) with the levels highest in subjects with chronic-progressive disease and lowest in subjects on ART. The horizontal lines at each variable indicate the mean (A). However, differences among subjects with HIV-1 infection were not statistically significant. We observed a strong correlation of sE-cadherin levels and viral load among the subjects with chronic HIV-1 infection (B; P = .004, R = 0.7) and a negative association of CD4+ T-cell count (in cells/mL) and sE-cadherin plasma levels (C). This correlation, however, did not reach statistical significance (P = .05, R = −0.51).
sE-cadherin levels in different stages of HIV-1 infection. The levels of sE-cadherin in the plasma were assessed by ELISA and are reported (in pg/mL). sE-cadherin levels were compared in HIV-negative subjects (n = 16), HIV-positive subjects on ART (n = 21), elite controllers (n = 14), and subjects with chronic-progressive HIV-1 infection (“progressors”; n = 15). sE-cadherin levels were significantly higher in all HIV-1 infected subjects compared with HIV-1 negative subjects (ART, P = .0002; elite controllers and progressors, P < .0001) with the levels highest in subjects with chronic-progressive disease and lowest in subjects on ART. The horizontal lines at each variable indicate the mean (A). However, differences among subjects with HIV-1 infection were not statistically significant. We observed a strong correlation of sE-cadherin levels and viral load among the subjects with chronic HIV-1 infection (B; P = .004, R = 0.7) and a negative association of CD4+ T-cell count (in cells/mL) and sE-cadherin plasma levels (C). This correlation, however, did not reach statistical significance (P = .05, R = −0.51).
Redistribution of E-cadherin in the intestinal mucosa of subjects with chronic HIV-1 infection
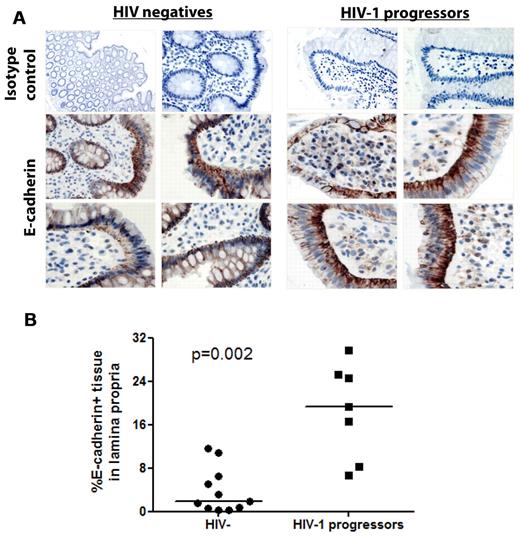
Previous reports have shown a profound and persistent depletion of CD4+ T cells in the gastrointestinal tissue starting in the earliest stages of acute HIV-1 infection.26,42-44 Moreover, studies have indicated that this depletion is accompanied by a sustained loss of epithelial integrity allowing for microbial translocation and ongoing immune activation.20 We therefore hypothesized that the disruption of intestinal epithelial integrity in HIV-1 infection also results in a redistribution of E-cadherin in the tissue and that this may be the source of the elevated levels of sE-cadherin detected in the plasma.
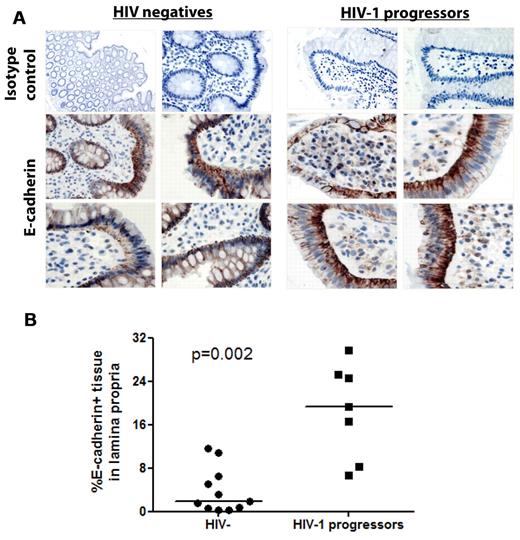
We therefore first compared compartmental differences in E-cadherin levels in plasma and gastrointestinal tissue of the same subjects. Strikingly, E-cadherin levels measured within the gastrointestinal mucosa were > 100 000-fold higher compared with levels in the plasma, suggesting a gradient between plasma and gastrointestinal tissue (supplemental Figure 5). We next assessed whether differences exist in E-cadherin mucosal distribution between HIV-1− and HIV-1+ persons. Endoscopic biopsies were obtained from the colon of 11 HIV-1–uninfected subjects and 7 subjects with chronic untreated HIV-1 infection, and specimens were stained by immunohistochemistry for E-cadherin (Figure 4A). In the HIV-negative subjects, E-cadherin was primarily localized in the single-cell mucosal epithelial layer, consistent with its function as an epithelial adhesion molecule. In contrast, in patients with chronic untreated HIV-1 infection, E-cadherin staining was detected in cells within the lamina propria, often diffusely or within intracytoplasmic vesicles. Quantitation of E-cadherin staining within the lamina propria by image analysis software showed that a significantly greater percentage of cells within the lamina propria stained for E-cadherin in HIV+ persons than in uninfected controls (P = .002 Mann-Whitney; Figure 4B). Confocal image analysis primarily showed internalization of sE-cadherin within CD19+ B cells and CD68+ monocytes/macrophages within the lamina propria but not CD3+ T cells (supplemental Figure 6). These results suggest that the disruption of intestinal mucosal integrity that accompanies HIV-1 infection leads to a substantial redistribution of E-cadherin within the gastrointestinal epithelium which then results in increased solubilization of the molecule. Because of its close proximity to the source, local concentrations within the gastrointestinal tissue are significantly higher than those systemically. Given the abundant expression of E-cadherin within the intestinal mucosa, it therefore is reasonable that this may be the primary source of elevated plasma levels of sE-cadherin.
Distribution of E-cadherin in the gastrointestinal mucosa. Pinch biopsies from the colon of 11 HIV-1 negative and 7 chronically infected HIV-1 subjects with progressive disease not on ART were obtained and stained for E-cadherin by immunohistochemistry (brown) and counterstained with hematoxylin. (A) Four representative immunohistochemical stainings from the intestine of HIV-negative and chronic untreated HIV-1+ subjects along with staining with an isotype control. E-cadherin in normal human colon is primarily localized to the mucosal epithelium and epithelial basement membrane, whereas redistribution of E-cadherin is visible in the lamina propria of HIV-1–infected subjects. Quantitation of E-cadherin staining was performed on 5 representative regions of the lamina propria from each section. The horizontal lines at each variable indicate the mean (B). The area of positive E-cadherin staining in the lamina propria was quantitated and found to be significantly higher in subjects with HIV-1 infection than in HIV-1–uninfected subjects (P = .002).
Distribution of E-cadherin in the gastrointestinal mucosa. Pinch biopsies from the colon of 11 HIV-1 negative and 7 chronically infected HIV-1 subjects with progressive disease not on ART were obtained and stained for E-cadherin by immunohistochemistry (brown) and counterstained with hematoxylin. (A) Four representative immunohistochemical stainings from the intestine of HIV-negative and chronic untreated HIV-1+ subjects along with staining with an isotype control. E-cadherin in normal human colon is primarily localized to the mucosal epithelium and epithelial basement membrane, whereas redistribution of E-cadherin is visible in the lamina propria of HIV-1–infected subjects. Quantitation of E-cadherin staining was performed on 5 representative regions of the lamina propria from each section. The horizontal lines at each variable indicate the mean (B). The area of positive E-cadherin staining in the lamina propria was quantitated and found to be significantly higher in subjects with HIV-1 infection than in HIV-1–uninfected subjects (P = .002).
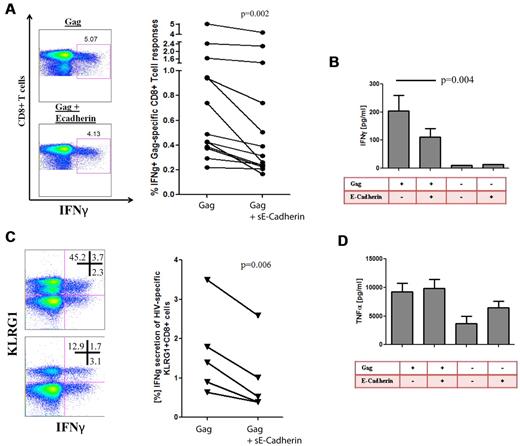
sE-cadherin inhibits HIV-1–specific CD8+ T cell cytokine secretion
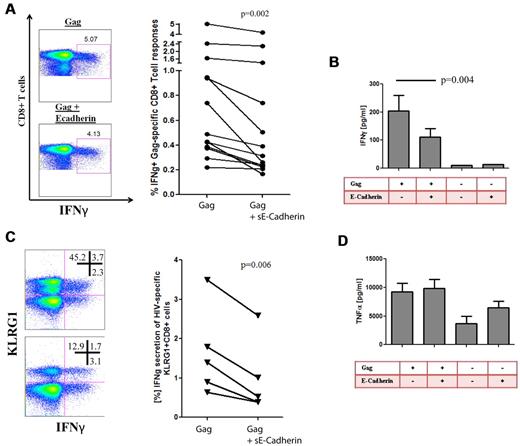
Previous studies have shown that KLRG1 ligation partially leads to decreased functionality of T cells. It has been shown that the inhibitory effect of KLRG1 is by active recruitment of SHIP-1 and SHP-2 and leads to a defective phosphorylation of the signaling kinase Akt(Ser273).29,45 We therefore hypothesized that the up-regulation of KLRG1 on CD8+ T cells results in an increased susceptibility of virus-specific CD8+ T cells to inhibition by sE-cadherin. We first confirmed that sE-cadherin was able to induce signaling events through KLRG1 ligation by analysis of Akt(Ser273) phosphorylation as previously described.29 Indeed, phosphorylation of Akt(Ser273) after stimulation was significantly down-regulated on sE-cadherin ligation (P = .02; supplemental Figure 7). Next, we assessed in PBMCs of subjects with chronic HIV-1 infection whether the presence or absence of increasing amounts of recombinant sE-cadherin lead to differences in their ability to secrete IFN-γ upon HIV-1 Gag peptide stimulation. Interestingly, sE-cadherin significantly abrogated the IFN-γ secretion of CD8+ T cells measured by intracellular cytokine staining (P = .002; Figure 5A) or Luminex (P = .004; Figure 5B) that was more pronounced on KLRG1+ CD8+ T cells (P = .006; Figure 5C) but also lead to a decrease of KLRG1 expression on E-cadherin ligation, supporting a direct interaction between KLRG1 and E-cadherin. Consistent with our previous observation, KLRG1+ CD8+ T cells were not capable of producing TNF-α in significant amount, and there were no changes in TNF-α secretion in response to Gag stimulation in the presence or absence of sE-cadherin (Figure 5D). Titrations of sE-cadherin down to a concentration of 10 pg/mL in serum-free media showed that even low concentrations of sE-cadherin can modulate the functionality of HIV-1–specific CD8+ T cells (data not shown). Treatment with sE-cadherin that was heat inactivated did not show any change in cytokine secretion compared with media control, and sE-cadherin had minimal toxicity as determined by stable levels of the apoptosis marker annexin V on CD8+ T cells after treatment with 1 μg/mL (data not shown).
HIV-1–specific CD8+ T-cell responses are impaired in cytokine production in the presence of sE-cadherin. Intracellular cytokine staining was used to determine whether the presence of sE-cadherin inhibits the function of Gag-specific CD8+ T cells. (A) Example of a Gag-specific CD8+ T-cell response in the presence or absence of sE-cadherin as well as the cumulative differences of all studied subjects with chronic HIV-1 infection (n = 13). In the presence of sE-cadherin, Gag-specific IFN-γ responses were significantly impaired (P = .002 paired t test). The numbers in each quadrant represent the % frequency of the parent. Similar results were obtained when assessing IFN-γ responses by Luminex (P = .004; B). Reduction in IFNγ secretion on E-cadherin ligation was most pronounced on KLRG1+ CD8+ T cells and also induced a KLRG1 down-regulation. The numbers in each quadrant represent the % frequency of the parent (C). However, no differences were observed for the TNF-α responses in the presence or absence of sE-cadherin (P = ns; D).
HIV-1–specific CD8+ T-cell responses are impaired in cytokine production in the presence of sE-cadherin. Intracellular cytokine staining was used to determine whether the presence of sE-cadherin inhibits the function of Gag-specific CD8+ T cells. (A) Example of a Gag-specific CD8+ T-cell response in the presence or absence of sE-cadherin as well as the cumulative differences of all studied subjects with chronic HIV-1 infection (n = 13). In the presence of sE-cadherin, Gag-specific IFN-γ responses were significantly impaired (P = .002 paired t test). The numbers in each quadrant represent the % frequency of the parent. Similar results were obtained when assessing IFN-γ responses by Luminex (P = .004; B). Reduction in IFNγ secretion on E-cadherin ligation was most pronounced on KLRG1+ CD8+ T cells and also induced a KLRG1 down-regulation. The numbers in each quadrant represent the % frequency of the parent (C). However, no differences were observed for the TNF-α responses in the presence or absence of sE-cadherin (P = ns; D).
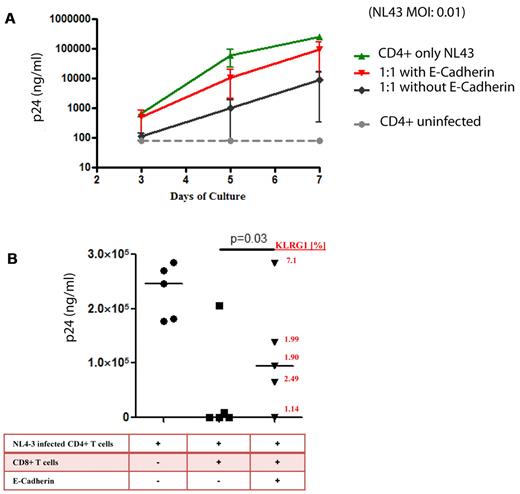
sE-cadherin inhibits HIV-1–specific antiviral activity of CD8+ T cells
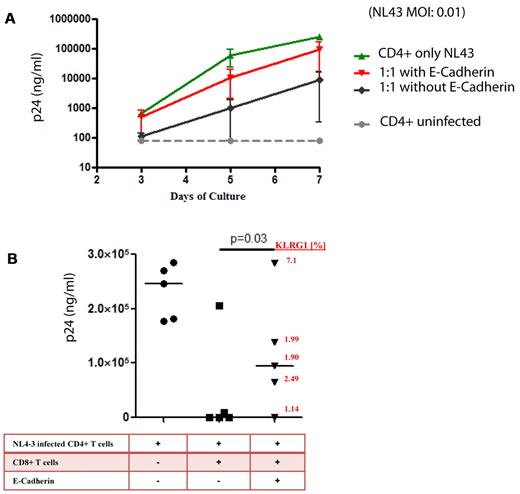
The ability of CD8+ T cells to kill virally infected cells is a hallmark of CTL immunologic function. We therefore also examined the effect of sE-cadherin on more complex antiviral functions such as cell lysis and viral inhibition in vitro. When CD4+ T cells from a chronically HIV-1–infected subject were isolated and infected with HIV-1NL4-3, robust viral replication was measured by p24 ELISA over the course of a 7-day viral culture (Figure 6A). Addition of autologous CD8+ T cells with known inhibitory capacity in a 1:1 effector-to-target ratio to the culture resulted in an inhibition of HIV-1 replication by > 2 logs. This viral inhibition was significantly impaired by the addition of sE-cadherin when tested in multiple HIV-1 chronically infected persons (Figure 6B). No effect was seen when heat-denatured sE-cadherin was added (data not shown). Interestingly, although the effect of sE-cadherin was relatively consistent in the viral inhibition assay, one patient who failed to show an effect of sE-cadherin on reducing HIV-1–specific CD8+ T-cell function also showed little expression of KLRG1 on tetramer-positive CD8+ T cells (Figure 6B).
E-cadherin reduces inhibition of HIV-1 replication by virus-specific CD8+ T cells. We choose subjects with known strong CD8+ T-cell responses to assess the effect of sE-cadherin on their inhibitory capacity. Bulk CD8 T cells directly isolated from peripheral blood by positive selection suppressed R5 replication in autologous CD4 T cells at a 1:1 ratio of CD8 to CD4 T cells. (A) The typical viral growth curve of NL4-3–infected CD4+ T cells in all cultures (green). The addition of autologous bulk CD8+ T cells lead to robust > 2 log viral inhibition. However, the addition of recombinant E-cadherin to the culture lead to an almost complete abrogation of the inhibitory effect of CD8+ T cells and the viral outgrowth being similar to viral outgrowth in absence of CD8+ T cells. (B) The effect of viral inhibition of bulk CD8+ T cells in the presence or absence of soluble E-cadherin in 5 different elite controllers is summarized. Viral replication was on average 232 072 ± 50 288 p24 pg/mL in the absence of CD8+ T cells, but abrogated to on average 42 903 ± 90 868 p24 pg/mL in the presence of bulk CD8+ T cells. However, the addition of sE-cadherin diminished the inhibitory effect of CD8+ T cells to 106 028 ± 115 919 p24 pg/mL. The strength of this effect was in relation to the expression of KLRG1 on the cell surface of CD8+ T cells after the resting period (red numbers). The horizontal line at each variable indicate the mean.
E-cadherin reduces inhibition of HIV-1 replication by virus-specific CD8+ T cells. We choose subjects with known strong CD8+ T-cell responses to assess the effect of sE-cadherin on their inhibitory capacity. Bulk CD8 T cells directly isolated from peripheral blood by positive selection suppressed R5 replication in autologous CD4 T cells at a 1:1 ratio of CD8 to CD4 T cells. (A) The typical viral growth curve of NL4-3–infected CD4+ T cells in all cultures (green). The addition of autologous bulk CD8+ T cells lead to robust > 2 log viral inhibition. However, the addition of recombinant E-cadherin to the culture lead to an almost complete abrogation of the inhibitory effect of CD8+ T cells and the viral outgrowth being similar to viral outgrowth in absence of CD8+ T cells. (B) The effect of viral inhibition of bulk CD8+ T cells in the presence or absence of soluble E-cadherin in 5 different elite controllers is summarized. Viral replication was on average 232 072 ± 50 288 p24 pg/mL in the absence of CD8+ T cells, but abrogated to on average 42 903 ± 90 868 p24 pg/mL in the presence of bulk CD8+ T cells. However, the addition of sE-cadherin diminished the inhibitory effect of CD8+ T cells to 106 028 ± 115 919 p24 pg/mL. The strength of this effect was in relation to the expression of KLRG1 on the cell surface of CD8+ T cells after the resting period (red numbers). The horizontal line at each variable indicate the mean.
Discussion
In this study we present data supporting a novel model in which 2 distinct pathways lead to the functional inhibition of HIV-1–specific CD8+ T cells and contribute to the lack of viral containment. Repetitive antigenic stimulation of CD8+ T cells in HIV-1–infected subjects with persistent high viral load results in the up-regulation of the inhibitory molecule KLRG1 on virus-specific CD8+ T cells. These HIV-1+ subjects concomitantly have a loss of gut mucosal epithelial integrity and increased levels of plasma sE-cadherin. The interaction of KLRG1+ HIV-1–specific CD8+ T cells with sE-cadherin can then lead to a significant impairment of antiviral functions of these cells on antigen engagement.
Virus-specific CD8+ T cells have been shown to be able to control viral replication. However, in chronic persistent viral infection, repetitive antigenic stimulation of these cells results in increasing exhaustion, which is accompanied by defects in antiviral cytokine secretion and proliferation, as well as the up-regulation of inhibitory receptors.3,4,6 The exact functional consequences of the accumulation of several inhibitory receptors on the surface of virus-specific CD8+ T cells are unknown, but it is thought that a finely tuned interplay of the different inhibitory signals is important in modulating their antiviral function. This is underlined by our finding that the expression of KLRG1 on virus-specific CD8+ T cells is not correlated with the expression of PD-1 or CTLA-4 but is correlated with the expression of lymphocyte activation gene-3 and CD57. These inhibitor pathways probably have complex interactions that are both independent and interdependent.
It has been previously reported that the interaction of KLRG1 and E-cadherin inhibits T- and natural killer–cell function.14,15 These studies suggested that KLRG1 increases the activation threshold of KLRG1+ lymphocytes in tissues expressing cadherins to prevent immunopathology. In the absence of chronic viral infections E-cadherin within the intestinal mucosa may help to balance inflammatory responses and to prevent immune disorders. In the setting of HIV-1 infection, however, concomitant disruption of mucosal integrity releases sE-cadherin, which then can inhibit both local and systemic immune function. The presence of sE-cadherin substantially abrogated the antiviral IFN-γ responses of the HIV-1–specific CD8+ T cells in our study. In contrast, we did not observe any effect of sE-cadherin on the production of TNF-α in line with the finding that KLRG1+ CD8+ T cells do not produce large amounts of TNF-α. In addition, sE-cadherin also led to a significant impairment in the ability of CD8+ T cells to inhibit viral replication. These are probably critical functions in vivo, suggesting that E-cadherin can affect the primary antiviral functions of CD8+ T cells.
Previous studies have shown that the gastrointestinal tract is one of the main sites of viral replication, and, even in the presence of highly active antiviral therapy, low levels of viral replication persist.46 Our results show a redistribution of E-cadherin within the intestinal mucosa of patients with chronic HIV-1 infection from the epithelium to regions throughout the lamina propria. Local concentrations of E-cadherin within the lamina propria were several logs higher than those found systemically, suggesting that a gradient exists between tissue and plasma. Although we were not able to measure the direct interaction of E-cadherin and KLRG1+ CD8+ T cells directly in the gastrointestinal tract, the concentration gradient suggests that local inhibition of HIV-specific responses in gut-associated lymphoid tissue might be substantially higher than seen systemically. This observation could be critical to the pathogenesis of HIV infection, given the central role of gut-associated lymphoid tissue as the primary reservoir of viral replication and latency.
In conclusion, we have demonstrated a novel mechanism in which HIV-1–specific CD8+ T cells up-regulate the inhibitory receptor KLRG1 on repetitive antigenic stimulation. Simultaneously, we demonstrate a significant redistribution of E-cadherin within the intestinal mucosa during HIV-1–associated disruption of epithelial integrity and an increase in sE-cadherin in the plasma. This sE-cadherin can then lead to a direct and substantial abrogation of antiviral functions of HIV-1–specific CD8+ T cells. This represents a novel mechanism by which HIV-1 immune pathology in the intestinal mucosa may directly contribute both to local and systemic T-cell dysfunction. In addition, disruption of E-cadherin/KLRG1 signaling may represent a novel interventional target that may help reverse immune exhaustion. Modulation of T-cell functions by liberated adhesion molecules represent an interesting and original concept of immune pathogenesis, which may play an important role in diseases other than HIV-1 infection. sE-cadherin is elevated in the setting of gastrointestinal cancers and has been described as a predictive marker for invasive gastrointestinal disease. This specific interaction may therefore also play an important role in CD8+ T-cell responsiveness against tumors that disrupt epithelial tissues.41
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Danny Douek (Vaccine Research Center, NIH, NIAID) for his help with the LPS and sCD14 assay.
The work was supported by NIH (grant R01-AI50429), the Bill and Melinda Gates Foundation, and the Susan and Philip T. Ragon Foundation.
National Institutes of Health
Authorship
Contribution: H.S., D.S.K., and M.A. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; T.C., S.J.R., J.J.C., D.A.M, D.E.K., and B.D.W. contributed to the design of experiments and interpretation of data; A.P. performed and analyzed flow cytometry experiments; M.N.A. performed immunofluorescent staining; I.T. and A.P.-T. provided experimental samples; and M.F., M.F.C., K.L., B.J., K.T., J.S.J., J.L., and Z.B. performed experiments and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hendrik Streeck, Ragon Institute of MGH, MIT and Harvard, 149 13th St, Boston, MA 02129; e-mail: hstreeck@partners.org.
References
Author notes
H.S. and D.S.K. contributed equally to this study.