Abstract
Whereas it is generally perceived to be harmful, enhanced coagulation activation can also convey salutary effects. The high prevalence of the prothrombotic factor V Leiden (FVL) mutation in whites has been attributed to a positive selection pressure (eg, resulting from reduced blood loss or improved survival in sepsis). The consequences of enhanced coagulation activation, as observed in FVL carriers, on microvascular diabetic complications remain unknown. We therefore investigated the role of FVL in diabetic nephropathy. In heterozygous or homozygous diabetic FVL mice, albuminuria and indices of diabetic nephropathy were reduced compared with diabetic wild-type mice. This was associated with reduced glomerular apoptosis and preservation of podocytes in diabetic FVL-positive mice. In vitro, low-dose thrombin (50pM) prevented, whereas high-dose thrombin (20nM) aggravated, glucose-induced apoptosis in podocytes. In diabetic patients, the FVL mutation, but not the plasminogen activator inhibitor-1 4G/5G polymorphism, is associated with reduced albuminuria, which is consistent with a nephroprotective role of low but sustained thrombin generation. Consistently, anticoagulation of diabetic FVL-positive mice with hirudin abolished the nephroprotective effect. These results identify a nephroprotective function of low but sustained thrombin levels in FVL carriers, supporting a dual, context-dependent function of thrombin in chronic diseases.
Introduction
The coagulation system provides an “on-demand” vascular repair system. After vascular injury, the coagulation system is activated, inducing fibrin formation and platelet activation, thus sealing the vascular leak and initiating a healing process leading in most cases to a restitutio ad integrum. As is evident in patients with severe hemophilia, this beneficial function of the hemostatic system is required for normal survival. Enhanced coagulation activation, however, is generally assumed to be disadvantageous for an individual's health, because some genetic polymorphisms associated with increased thrombin generation mediate an increased risk of venous thrombosis.1 Considering the impending health risks associated with prothrombotic genetic polymorphisms, the high prevalence of some of them, in particular the factor V Leiden (FVL) mutation, led to the assumption that these genetic polymorphisms must be coupled with a positive selection pressure during evolution.2
The FVL mutation is a missense mutation in the factor V gene (R506Q), resulting in the resistance of activated factor V to inactivation by activated protein C (aPC). The prevalence of the FVL mutation in whites is 4%-6%.1 The potential benefits associated with FVL include a higher embryonic implantation rate, reduced puerperal maternal blood loss, and improved survival from severe infections.3-5 The latter has been a matter of debate, because not all studies were able to reproduce the improved survival of FVL carriers in sepsis.6-11 We have previously shown that the presence of the FVL mutation results in a partial benefit during atherogenesis, evoking larger but more stable plaques.12 This implies that FVL-mediated salutary effects may provide a health benefit, not only in acute health threats such as blood loss or severe infection, but possibly also in chronic diseases.
Diabetic nephropathy, a chronic complication of diabetes mellitus, is now the most frequent cause of terminal kidney failure in industrialized countries.13 Diabetic nephropathy is associated with a poor prognosis for affected patients, and constitutes a significant financial burden for health-care systems. Hypercoagulability in diabetic patients is well established, but its relevance for disease progression (eg, for diabetic nephropathy) remains poorly understood. Several studies have evaluated the efficacy of heparins in diabetic nephropathy. These research efforts, however, were not motivated by the anticoagulant properties of heparins. Based on the Steno hypothesis, these studies evaluated whether heparins could correct the altered sulfation pattern of glycosaminoglycan side chains and heparan sulfate proteoglycans in the glomerular filtration barrier.14,15 The divergent results obtained in these studies, including worsening of albuminuria in heparin-treated diabetic rats in one study, may reflect the different properties of the glycosaminoglycans used.14,16-20 More recently, we established that endothelial dysfunction with loss of thrombomodulin (TM)–dependent protein C (PC) activation aggravates diabetic nephropathy in mice.20 Impaired function of endothelial TM provoked hypercoagulability in diabetic mice; however, the aggravation of diabetic nephropathy was not caused by enhanced coagulation activation per se, but by the lack of aP-dependent cytoprotective signaling. APC suppresses glucose-induced apoptosis in endothelial cells and podocytes through a receptor-dependent mechanism and prevents glomerular apoptosis, albuminuria, and morphologic hallmarks of diabetic nephropathy in mice.20
The FVL mutation disrupts the same anticoagulant pathway. However, unlike the dysfunctional TM mutant (TMPro/Pro), which impairs PC activation, FVL interferes with this pathway at the substrate level, leading to aPC resistance without impeding PC activation. Therefore, it remains to be shown whether the FVL mutation has the same deleterious consequences as the TMPro/Pro mutation in diabetic nephropathy. Alternatively, FVL may mediate a salutary effect in diabetic nephropathy through an unknown mechanism. In the present study, we investigated the effect of the FVL mutation on diabetic nephropathy and, surprisingly, found that the presence of the FVL mutation reduced albuminuria in experimental murine diabetic nephropathy and in human type 1 and type 2 diabetic patients. We have therefore identified a new function of FVL in the modification of diabetic nephropathy, which adds to the research regarding the salutary effects associated with low but sustained coagulation activation observed in FVL carriers.
Methods
Reagents
For reagent details, see supplemental Methods section (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patients and genotyping of human DNA samples
Clinical data and DNA samples from 200 type 1 and 350 type 2 white diabetic patients were analyzed. Patients were consecutively enrolled between January 1998 and October 2008 from the outpatient clinic of the Department of Endocrinology (University of Heidelberg, Heidelberg, Germany). All patients gave informed consent and the study was approved by the ethical committee of the Department of Medicine, University of Heidelberg. Diabetes was diagnosed according to World Health Organization criteria. Analyses were performed in a blinded cross-sectional approach. Patients with a diabetes duration < 10 years or with terminal kidney failure were excluded. Likewise, patients receiving long-term anticoagulant treatment (eg, with heparins or vitamin K antagonists) were excluded. Because the study was performed as a monocentric study, patients who had relatives with diabetes who were also treated in the recruiting center were also excluded. For comparison of plasma creatinine or albuminuria, the average of the last 3 available determinations was used. Albuminuria measurements performed in patients with concomitant urinary infections were excluded.
Genomic DNA was prepared from peripheral blood using the QIAmp Blood Kit (QIAGEN) according to the manufacturer's instructions. Genetic polymorphisms in the factor V (FV) and the plasminogen activator inhibitor-1 (PAI-1) genes were detected by fluorescent resonance energy transfer probes with the LightCycler (Roche Molecular Biochemicals). DNA fragments were amplified using the primers FV-f 5′-TGC CCA GTG CTT AAC AAG ACC A-3′ and FV-r 5′-CTT GAA GGA AAT GCC CCA TTA-3′ or PAI-f 5′-AGC CAG ACA AGG TTG TTG ACA C-3′ and PAI-r 5′-CAG AGG ACT CTT GGT CTT TCC C-3′, respectively. The following hybridization probes were used: FV-FL 5′-GGC GAG GAA TAC AGG TAT-3′ and FV-LCred 5′-TGT CCT TGA AGT AAC CTT TCA GAA ATT CTG-3′ or PAI-FL: 5′-TGA CTC CCC CAC GTG TCC-3′, PAI-LCred: 5′-ACT CTC TCT GTG CCC CTG AGG GCT CT-3′. Primers were obtained from TIB MOLBIOL. An annealing temperature of 55°C (FV) or 57°C (PAI-1) was used.
Mice
Heterozygous FVLr/q and homozygous FVLq/q mice have been described previously.21,22 In the current study, we used littermates in which at least 98% of the genetic background was C57BL/6 derived. We injected a subset of diabetic mice (see “Induction of diabetes using STZ”) either subcutaneously with hirudin (1.5 mg/kg body weight) or saline once daily starting 3 weeks after the last streptozotocin (STZ) injection until 1 day before analysis (week 26). Animal experiments were conducted following the standards and procedures approved by the local animal care and use committee (Regierungspräsidium Karlsruhe, Germany).
Induction of diabetes using STZ
Diabetes was induced by intraperitoneal administration of 60 mg/kg STZ freshly dissolved in 0.05M sterile sodium citrate, pH 4.5, on 5 successive days in 8-week-old mice. Mice were considered diabetic if blood glucose levels were above 300 mg/dL 16-25 days after the last STZ injection. Blood glucose levels were determined in blood samples from the tail vein using ACCU-CHEK glucose sticks. In the first 3 weeks after the onset of diabetes, blood glucose values were measured 3 times per week, and after 3 weeks they were measured once a week. Mice displaying blood glucose levels above 500 mg/dL received 1-2 U of insulin (Lantus) to avoid excessively and potentially lethal hyperglycemia. We obtained blood and tissue samples after 26 weeks of persistent hyperglycemia in diabetic mice. Age-matched littermates served as controls. At the time of analysis, the weights of the mice and of their organs were recorded.
Determination of albuminuria
The day before blood sample collection and tissue preparation, individual mice were placed in metabolic cages and 24-hour urine samples were collected. We determined urine albumin using a mouse albumin ELISA according to the manufacturer's instructions, and urine creatinine using a commercially available assay of a modified version of the Jaffe method (X-Pand automated platform; Siemens).23
Analyses of TAT complexes
We determined thrombin-antithrombin (TAT) complexes using a TAT ELISA according to the manufacturer's instructions, as described previously.20
Histology and immunohistochemistry
Immunohistochemical and terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) analyses were performed essentially as described previously20 (see supplemental Methods for further details).
Immunoblotting and immunoprecipitation
Immunoblotting was performed essentially as described previously20 (see supplemental Methods for further details).
Cell culture
Determination of apoptosis
Human and mouse podocytes were serum starved overnight in serum-free medium and pretreated with thrombin for 1 hour before the addition of high glucose (30mM). Because of its short half-life, thrombin was added after every 12 hours throughout the experiment. After 48 hours of glucose treatment, cells were fixed in 4% neutral-buffered formalin, washed in 1× PBS, and apoptosis was determined using the TUNEL method, as described previously20 (see supplemental Methods for further details).
Activated PC in vivo capture assay
Mice were anesthetized and human PC (20 μg in 100 μL of 1× PBS) or 1× PBS (100 μL per mouse) was injected via the tail vein. After 10 minutes, blood samples were collected from the vena cava into 0.38% sodium citrate and 50mM benzamidine HCl (final concentrations). Human aPC was captured from these plasma samples using an antibody highly specific for human aPC (HAPC 1555), and the activity of the captured human PC was determined using the chromogenic substrate Spectrozyme PCa, as described previously.20
Statistical analyses
The data are summarized as the means ± SEM. Statistical analyses were performed using the Student t test or ANOVA. Correction for multiple testing was performed. StatistiXL software Version 1.6 (http://www.statistixl.com) was used for all statistical analyses. Statistical significance was accepted as P < .05.
Results
FVL protects against diabetic nephropathy in mice
To evaluate the effect of the FVL mutation on diabetic nephropathy, we induced hyperglycemia using STZ in wild-type (FVwt), heterozygous (FVLr/q), and homozygous (FVLq/q) FVL mice. Mice were kept diabetic for 26 weeks, allowing us to evaluate the consequences of persistent hyperglycemia. Blood glucose concentrations and the amount of insulin required to avoid excessive and lethal hyperglycemia did not differ between groups (Figure 1A and data not shown), allowing for direct comparison of the diabetic mouse groups.
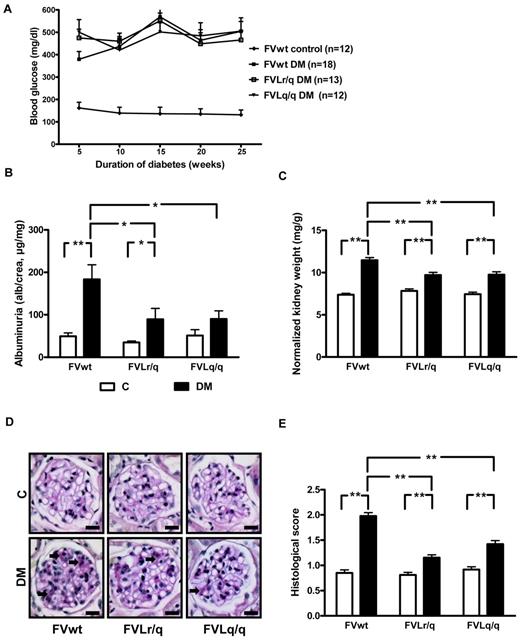
FVL ameliorates experimental diabetic nephropathy in mice. (A) Blood glucose concentrations in nondiabetic wild-type (FVwt control, n = 12), diabetic wild-type (FVwt DM, n = 18), diabetic heterozygous (FVLr/q DM, n = 13), or diabetic homozygous (FVLq/q DM, n = 12) FVL mice. Blood glucose levels did not differ between groups of diabetic mice. Albuminuria (B), normalized kidney weight (C), and extracellular matrix deposition as determined by PAS staining (D-E) in FVwt, FVLr/q, and FVLq/q mice without or with diabetes (n ≥ 10 for each group) are shown. These indices of diabetic nephropathy remained significantly lower in diabetic FVLr/q or FVLq/q mice. Exemplary images of PAS-stained histologic sections (D arrows, PAS-positive area) and bar graph summarizing results of histologic scores (E) (≥ 50 glomeruli of at least 7 different mice were analyzed). Scale bar indicates 15 μm (D). C indicates nondiabetic control mice (white bars); DM, diabetic mice (black bars); means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
FVL ameliorates experimental diabetic nephropathy in mice. (A) Blood glucose concentrations in nondiabetic wild-type (FVwt control, n = 12), diabetic wild-type (FVwt DM, n = 18), diabetic heterozygous (FVLr/q DM, n = 13), or diabetic homozygous (FVLq/q DM, n = 12) FVL mice. Blood glucose levels did not differ between groups of diabetic mice. Albuminuria (B), normalized kidney weight (C), and extracellular matrix deposition as determined by PAS staining (D-E) in FVwt, FVLr/q, and FVLq/q mice without or with diabetes (n ≥ 10 for each group) are shown. These indices of diabetic nephropathy remained significantly lower in diabetic FVLr/q or FVLq/q mice. Exemplary images of PAS-stained histologic sections (D arrows, PAS-positive area) and bar graph summarizing results of histologic scores (E) (≥ 50 glomeruli of at least 7 different mice were analyzed). Scale bar indicates 15 μm (D). C indicates nondiabetic control mice (white bars); DM, diabetic mice (black bars); means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
Persistent hyperglycemia resulted in marked and highly significant albuminuria in FVwt mice (183.4 μg/mg vs 49.5 μg/mg, P < .001; Figure 1B). Albuminuria was also increased in diabetic FVLr/q and FVLq/q mice, but compared with nondiabetic controls the increase was only of borderline (89.3 μg/mg, P = .046, for FVLr/q mice) or no significance (90.2 μg/mg, P = .06, for FVLq/q mice; Figure 1B). Albuminuria in diabetic FVLr/q or FVLq/q mice was significantly lower compared with diabetic FVwt mice (P = .02 and P = .02, respectively; Figure 1B).
Consistent with the observed changes in albuminuria, the increase in the normalized kidney weight was more pronounced in diabetic FVwt mice than in diabetic FVLr/q mice (11.5 μg/mg vs 9.7 μg/mg, P < .001; Figure 1C) or FVLq/q mice (11.5 μg/mg vs 9.8 μg/mg, P < .001; Figure 1C). However, FVL-positive mice were not completely protected against renal hypertrophy, and the increase in the normalized kidney weight remained significant in these mice compared with nondiabetic littermates (9.7 μg/mg vs 7.8 μg/mg, P < .001, for the FVLr/q mice and 9.8 μg/mg vs 7.5 μg/mg, P < .001, for the FVLq/q mice; Figure 1C).
Analyses of period acid-Schiff (PAS)–stained histologic sections also revealed an increase of an extracellular, PAS-positive matrix predominately in diabetic FVwt mice (histologic score 2.0 vs 0.9, P < .001; Figure 1D-E). In diabetic FVL-positive mice, the histologic score was significantly lower compared with diabetic FVwt mice (histologic score 2.0 vs 1.2 in diabetic FVLr/q mice, P < .001, and 2.0 vs 1.4 in diabetic FVLq/q mice, P < .001; Figure 1D-E). Again, the presence of the FVL mutation did not completely prevent extracellular matrix accumulation, resulting in a higher score compared with nondiabetic littermates (histologic score 1.2 vs 0.8 in FVLr/q mice, P < .001, and 1.4 vs 0.9 in FVLq/q mice, P < .001; Figure 1D-E). These data show that both the heterozygous and the homozygous FVL mutation ameliorates diabetic nephropathy in mice.
Reduced frequency of glomerular apoptosis in FVL-positive mice
Inhibition of glomerular apoptosis ameliorates diabetic nephropathy in mice.20 We therefore evaluated the frequency of apoptotic cells within renal glomeruli and the expression of apoptosis regulators. In diabetic FVwt mice, the frequency of apoptotic glomerular cells was markedly increased (3.5% vs 1.6% in nondiabetic FVwt mice, P < .001; Figure 2A-B). A tendency to increased apoptosis was also apparent in diabetic FVLr/q and FVLq/q mice, but the difference was nonsignificant (1.9% vs 1.5% in FVLr/q mice, P = .052; and 1.8% vs 1.6% in FVLq/q mice, P = .21; Figure 2A-B). The frequency of apoptotic cells within the glomeruli of diabetic FVLr/q or FVLq/q mice was significantly lower than that in diabetic FVwt mice (1.9% or 1.8% vs 3.5% in FVwt, P < .001 and P < .001, respectively; Figure 2A-B).
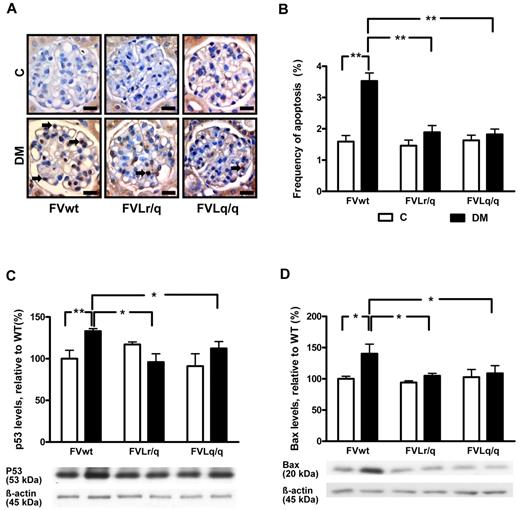
Reduced glomerular apoptosis in FVL mice. (A-B) Frequency of apoptosis in glomeruli as determined by TUNEL assay. Apoptosis was more frequent in glomeruli of diabetic wild-type mice (FVwt, arrows) than in diabetic heterozygous (FVLr/q, DM, arrows) or homozygous (FVLq/q, DM, arrows) FVL mice. Exemplary images of TUNEL stain (A) and bar graph (B) summarizing results. Brown indicates TUNEL-positive cells detected by HRP-DAB reaction; blue, hematoxylin counterstain; n ≥ 7 for each group. (C-D) Expression of apoptosis regulators in renal cortex extracts. Bar graph (top) and representative immunoblot (bottom) showing p53 (C) and Bax (D) expression in renal cortex tissue samples summarizing results (n ≥ 7 for each group). Scale bar indicates 15 μm (A). C indicates nondiabetic, control mice (white bars); DM, diabetic mice (black bars); means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
Reduced glomerular apoptosis in FVL mice. (A-B) Frequency of apoptosis in glomeruli as determined by TUNEL assay. Apoptosis was more frequent in glomeruli of diabetic wild-type mice (FVwt, arrows) than in diabetic heterozygous (FVLr/q, DM, arrows) or homozygous (FVLq/q, DM, arrows) FVL mice. Exemplary images of TUNEL stain (A) and bar graph (B) summarizing results. Brown indicates TUNEL-positive cells detected by HRP-DAB reaction; blue, hematoxylin counterstain; n ≥ 7 for each group. (C-D) Expression of apoptosis regulators in renal cortex extracts. Bar graph (top) and representative immunoblot (bottom) showing p53 (C) and Bax (D) expression in renal cortex tissue samples summarizing results (n ≥ 7 for each group). Scale bar indicates 15 μm (A). C indicates nondiabetic, control mice (white bars); DM, diabetic mice (black bars); means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
Expression levels of apoptosis regulators were analyzed in renal cortex tissue samples. Expression of the proapoptotic proteins p53 and Bax was significantly higher in diabetic FVwt mice (132.4% and 140.3%, P < .001 and P = .02, respectively, compared with nondiabetic FVwt mice; Figure 2C-D). Conversely, expression of p53 and Bax remained normal in diabetic FVLr/q and FVLq/q mice (105.8% and 104.9%, P = .13 and P = .08, respectively, in FVLr/q mice and 95.9% and 108.7%, P = .17 and P = .12, respectively, in FVLq/q mice; Figure 2C-D). Expression levels of the antiapoptotic regulator Bcl-2 remained unaltered in all groups (data not shown). These observations establish that glomerular apoptosis is inhibited in the presence of the FVL mutation.
FVL mutation prevents hyperglycemia-induced podocyte loss
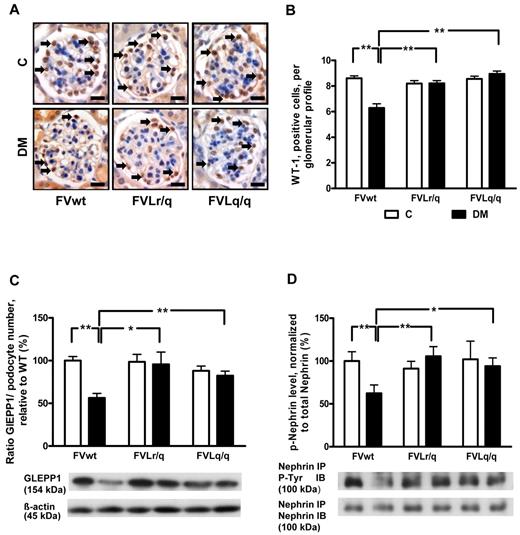
We analyzed podocyte numbers to evaluate whether the FVL mutation protects against hyperglycemia-induced podocyte loss. Immunohistochemical staining of podocytes using an antibody against Wilms tumor 1 (WT-1) showed a reduced podocyte number in diabetic FVwt mice (5.6 vs 8.6 in nondiabetic FVwt mice, P < .001; Figure 3A-B), but not in diabetic FVLr/q or FVLq/q mice (8.2% and 8.9%, P = .48 and P = .11, respectively; Figure 3A-B). Likewise, the expression of glomerular epithelial protein 1 (GLEPP1), a protein expressed specifically in podocytes within the kidney, was reduced only in diabetic FVwt mice (normalized for podocyte numbers: 56.3% vs 100.0% in nondiabetic FVwt mice, P < .001; Figure 3C and supplemental Figure 1A-B), but not in diabetic FVLr/q or FVLq/q mice (95.5% and 82.3%, P = .86 and P = .47, respectively; Figure 3C and supplemental Figure 1A-B).
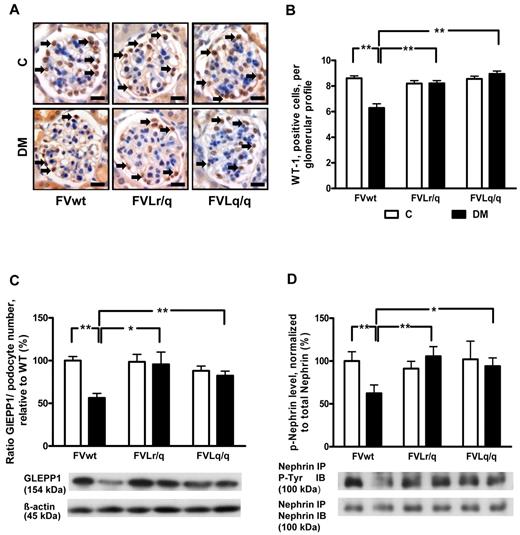
FVL protects against podocyte loss. (A-B) Podocyte number in glomeruli as determined by WT-1 immunohistochemical staining. Podocyte frequency was reduced in the glomeruli of diabetic wild-type mice (FVwt, arrows), but not in diabetic heterozygous (FVLr/q, arrows) or diabetic homozygous (FVLq/q, arrows) FVL mice. WT-1–positive cells detected by HRP-DAB reaction (brown) or hematoxylin counterstain (blue); n ≥ 7 for each group. (C) Bar graph (top, data normalized for podocyte numbers) and representative immunoblot (bottom) showing GLEPP1 expression in renal cortex tissue samples. GLEPP1 expression was reduced in diabetic wild-type, but not in diabetic FVL-positive mice. Actin immunoblot is shown as loading control (n ≥ 7 for each group). (D) Bar graph (top) and representative immunoblots of immunoprecipitates (bottom) showing nephrin tyrosine phosphorylation in renal cortex tissue samples. Nephrin tyrosine phosphorylation (normalized to total nephrin) is reduced in diabetic wild-type but not in diabetic FVL-positive mice. Bar graphs summarizing the results (n ≥ 7 for each group). Scale bar indicates 15 μm (A). C indicates nondiabetic, control mice (white bars); DM, diabetic mice (black bars); means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
FVL protects against podocyte loss. (A-B) Podocyte number in glomeruli as determined by WT-1 immunohistochemical staining. Podocyte frequency was reduced in the glomeruli of diabetic wild-type mice (FVwt, arrows), but not in diabetic heterozygous (FVLr/q, arrows) or diabetic homozygous (FVLq/q, arrows) FVL mice. WT-1–positive cells detected by HRP-DAB reaction (brown) or hematoxylin counterstain (blue); n ≥ 7 for each group. (C) Bar graph (top, data normalized for podocyte numbers) and representative immunoblot (bottom) showing GLEPP1 expression in renal cortex tissue samples. GLEPP1 expression was reduced in diabetic wild-type, but not in diabetic FVL-positive mice. Actin immunoblot is shown as loading control (n ≥ 7 for each group). (D) Bar graph (top) and representative immunoblots of immunoprecipitates (bottom) showing nephrin tyrosine phosphorylation in renal cortex tissue samples. Nephrin tyrosine phosphorylation (normalized to total nephrin) is reduced in diabetic wild-type but not in diabetic FVL-positive mice. Bar graphs summarizing the results (n ≥ 7 for each group). Scale bar indicates 15 μm (A). C indicates nondiabetic, control mice (white bars); DM, diabetic mice (black bars); means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
To investigate whether in addition to podocyte number, podocyte function may also be preserved in diabetic FVLr/q or FVLq/q mice, we next determined the tyrosine phosphorylation of nephrin, a podocyte transmembrane protein. Tyrosine phosphorylation regulates the interaction of nephrin with adaptor proteins such as Nck, modulating the actin cytoskeleton.25 Nephrin tyrosin phosphorylation (normalized to total nephrin levels) was markedly reduced in diabetic FVwt mice (62.3% vs 100.0% in nondiabetic FVwt mice, P = .004; Figure 3D), but not in diabetic FVLr/q or FVLq/q mice (105.6% and 93.5%, P = .74 and P = .69, respectively; Figure 3D). These data indicate that the FVL mutation protects against hyperglycemia-induced podocyte loss and dysfunction in mice.
FVL, but not the PAI-1 4G/5G polymorphism, is associated with reduced albuminuria in human diabetic patients
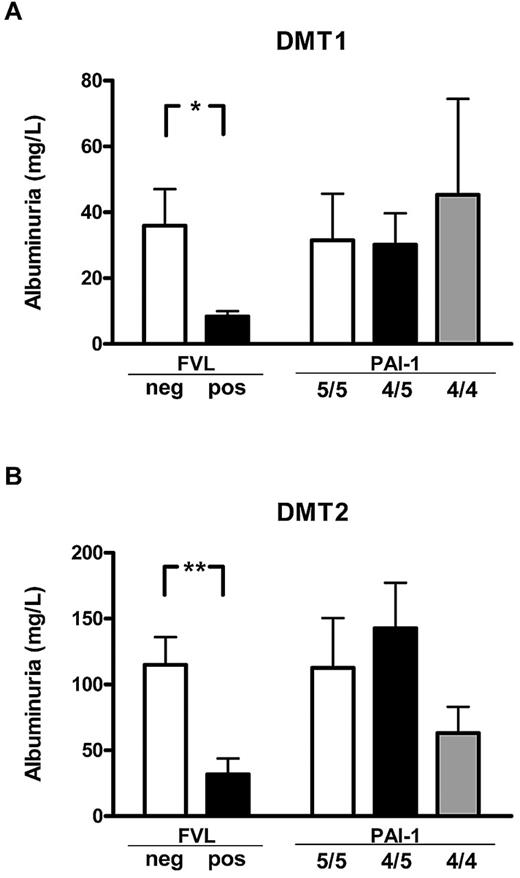
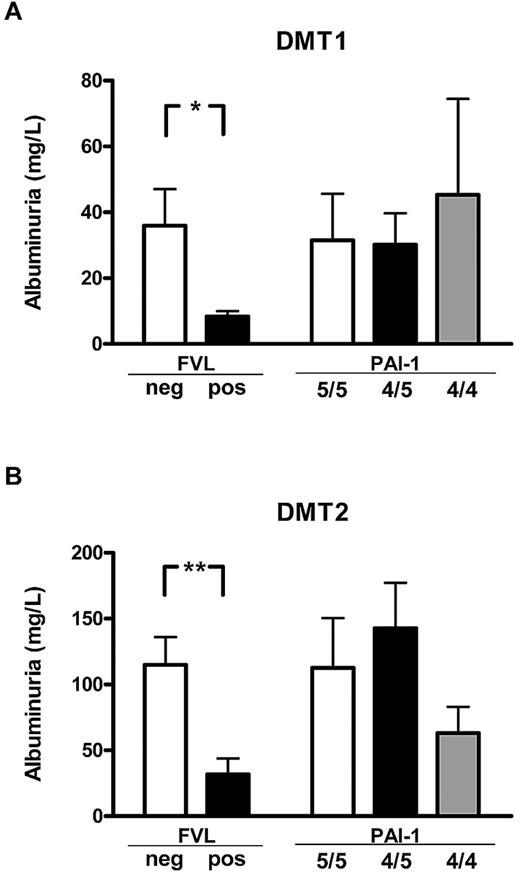
To determine the effect of FVL on diabetic nephropathy in human patients, we evaluated albuminuria in a cohort of diabetic patients with or without the FVL mutation. Clinical characteristics and DNA samples from 200 type 1 diabetic and 350 type 2 diabetic patients were prospectively collected from consecutive patients presenting in our outpatient clinic. Patients with or without the FVL mutation did not differ in regard to age, sex distribution, disease duration, HbA1c, plasma cholesterol or triglycerides, the presence of hypertension, or a current history of smoking (Table 1). No patient homozygous for the FVL mutation was identified, whereas 14 type 1 diabetic patients (7.0%) and 24 type 2 diabetic patients (6.8%) were heterozygous for the FVL mutation (FVL-positive). Albuminuria was significant lower both in type 1 and type 2 FVL-positive diabetic patients (8.4 ± 1.7 mg/L vs 36.0 ± 11.1 mg/L, P = .041, in type 1 diabetic patients; 31.7 ±12.0 mg/L vs 115.0 ± 21.0 mg/L, P = .003, in type 2 diabetic patients; Figure 4). No significant differences in plasma creatinine values were observed between type 1 and type 2 diabetic patients, although we observed a nonsignificant trend toward lower creatinine levels in FVL-positive type 2 diabetic patients (mean 1.10 mg/dL in FVL-negative patients vs 0.89 mg/dL in FVL-positive patients, P = .082). This reduction of albuminuria in FVL-positive diabetic patients is consistent with our observations in mice.
FVL heterozygosity ameliorates diabetic nephropathy in diabetic patients. Average albuminuria in diabetic patients with either type 1 (DMT1; A) or type 2 (DMT2; B) diabetes mellitus stratified according to the genetic FVL or PAI-1 4G/5G polymorphisms. Average albuminuria was lower in patients carrying the FVL mutation (FVL pos) than in patients not carrying the FVL mutation (FVL neg), but did not differ in patients stratified according to the PAI-1 polymorphism. Means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
FVL heterozygosity ameliorates diabetic nephropathy in diabetic patients. Average albuminuria in diabetic patients with either type 1 (DMT1; A) or type 2 (DMT2; B) diabetes mellitus stratified according to the genetic FVL or PAI-1 4G/5G polymorphisms. Average albuminuria was lower in patients carrying the FVL mutation (FVL pos) than in patients not carrying the FVL mutation (FVL neg), but did not differ in patients stratified according to the PAI-1 polymorphism. Means ± SEM are shown; *P < .05; **P < .01 (ANOVA).
The FVL mutation is associated with increased thrombin and fibrin generation.1 To determine whether the amelioration of albuminuria may dependent on increased thrombin or fibrin generation, we determined the PAI-1 4G/5G polymorphism in our patient cohort. Both polymorphisms are associated with increased fibrin formation, but only the FVL polymorphism is associated with increased thrombin formation. Patient groups stratified to the PAI-1 4G/5G polymorphism did not differ in regard to age, sex distribution, disease duration, HbA1c, plasma cholesterol or triglycerides, the presence of hypertension, or a current history of smoking (Table 1). No association between the PAI-1 polymorphism and albuminuria was observed. In particular, albuminuria in patients carrying the PAI-1 4G/4G polymorphism, which is associated with an increased risk of thrombosis but not with enhanced thrombin formation, was not significantly reduced (45.3 ± 29.1 mg/L vs 30.2 ± 9.5 mg/L or 31.5 ± 13.5 mg/L, P > .05 for both in type 1 diabetic patients; 63.1 ± 20.0 mg/L vs 142.7 ± 34.5 mg/L or 112.7 ± 37.7 mg/L, P > .05 for both in type 2 diabetes patients; Figure 4). The association between reduced albuminuria in diabetic patients with a genetic bias to enhanced thrombin generation (FVL-positive), but not in patients with a genetic bias to impaired fibrin dissolution (PAI-1 4G/4G) supports the notion that increased thrombin formation ameliorates albuminuria in diabetic patients.
Low-dose thrombin inhibits high-glucose–induced apoptosis in podocytes
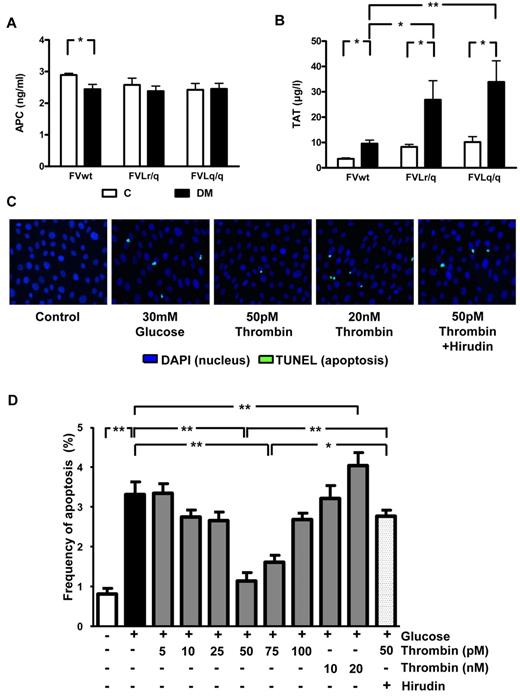
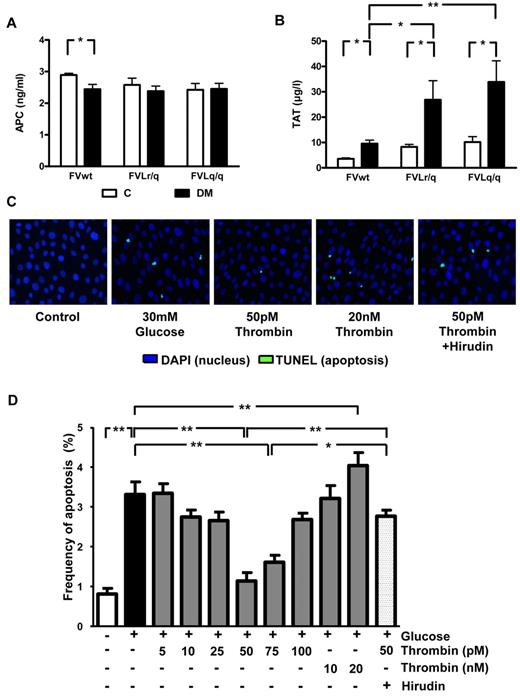
It has been previously speculated that the low but sustained activation of thrombin in FVL-positive carriers increases PC activation, resulting in cytoprotection.5 Consistently, the improved survival of lipopolysaccharide (LPS)–challenged mice was associated with increased in vivo PC activation. In contrast, in diabetic mice, the presence of the FVL mutation did not enhance in vivo PC activation (2.45 ng/mL in diabetic FVwt vs 2.36 ng/mL in diabetic FVLr/q or 2.42 ng/mL in diabetic FVLq/q mice; Figure 5A). Whereas no difference in in vivo PC activation was detected, the presence of the FVL mutation did increase plasma levels of TAT, a marker of thrombin generation. In nondiabetic mice, the presence of the FVL mutation roughly doubled the TAT level (8.3 μg/L, P = .038, and 10.2 μg/L, P = .005, in FVLr/q and FVLq/q mice, respectively, vs 3.6 μg/L in FVwt mice; Figure 5B). TAT levels further increased in diabetic FVLr/q and FVLq/q mice (26.9 μg/L, P = .048, and 33.9 μg/L, P = .008, vs 9.6 μg/L in diabetic FVwt mice; Figure 5B). Therefore, in mice with persistent hyperglycemia, the FVL mutation augments thrombin generation. Increased thrombin generation did not result in macroscopically detectable spontaneous thrombosis (data not shown).
Thrombin, but not PC activation, is enhanced in diabetic FVL mice and low-concentration thrombin protects glucose-stressed human podocytes. (A) In vivo aPC formation did not differ in diabetic FVwt, diabetic FVLr/q, or diabetic FVLq/q mice; n ≥ 7 for each group. (B) TAT levels in nondiabetic control mice or mice with long-term (26 weeks) hyperglycemia. Hyperglycemia increased TAT levels in wild-type (FVwt) and to a larger extent in heterozygous (FVLr/q) or homozygous (FVLq/q) FVL mice (n ≥ 10 for each group). (C-D) Apoptosis (green, TUNEL-positive) in cultured human podocytes exposed to glucose in the presence or absence of aPC (20nM) or thrombin (range, 5-20 nM). Representative images (C) with fluorescent-labeled nucleotides (green, TUNEL-positive) and Hoechst 33258 nuclear counter stain (blue) and bar graph (D) summarizing results of at least 3 independent experiments performed in duplicate; means ± SEM are shown. C indicates nondiabetic, control mice (white bars); DM, diabetic mice (black bars); *P < .05; **P < .01 (ANOVA).
Thrombin, but not PC activation, is enhanced in diabetic FVL mice and low-concentration thrombin protects glucose-stressed human podocytes. (A) In vivo aPC formation did not differ in diabetic FVwt, diabetic FVLr/q, or diabetic FVLq/q mice; n ≥ 7 for each group. (B) TAT levels in nondiabetic control mice or mice with long-term (26 weeks) hyperglycemia. Hyperglycemia increased TAT levels in wild-type (FVwt) and to a larger extent in heterozygous (FVLr/q) or homozygous (FVLq/q) FVL mice (n ≥ 10 for each group). (C-D) Apoptosis (green, TUNEL-positive) in cultured human podocytes exposed to glucose in the presence or absence of aPC (20nM) or thrombin (range, 5-20 nM). Representative images (C) with fluorescent-labeled nucleotides (green, TUNEL-positive) and Hoechst 33258 nuclear counter stain (blue) and bar graph (D) summarizing results of at least 3 independent experiments performed in duplicate; means ± SEM are shown. C indicates nondiabetic, control mice (white bars); DM, diabetic mice (black bars); *P < .05; **P < .01 (ANOVA).
Thrombin conveys concentration-dependent effects in endothelial cells. At a low dose (∼ 50pM) thrombin mediates protective effects, whereas at higher concentrations (> 100pM) thrombin activates endothelial cells, resulting in a proinflammatory phenotype.26 Considering the increased TAT plasma levels and the associated podocyte protection in FVL-positive mice, we hypothesized that thrombin dose dependently regulates podocyte apoptosis. To test this hypothesis, we used conditionally immortalized human and mouse podocytes, induced apoptosis using high glucose concentrations, and determined the effect of thrombin added at various concentrations (5-20nM). Exposure of human podocytes to glucose (30mM for 48 hours) induced apoptosis (3.3% vs 0.8% in untreated podocytes, P < .001; Figure 5C-D), which is consistent with the proapoptotic effect of glucose in murine podocytes.20 Preincubation with 50 or 75pM thrombin abolished the proapoptotic effect (1.1% or 1.6% vs 3.3% in untreated but glucose-stressed podocytes, P < .001 for both; Figure 5C-D), whereas lower or higher thrombin concentrations had no effect on or even significantly increased (20nM thrombin) apoptosis compared with glucose only (Figure 5C-D). The antiapoptotic effect observed with 50pM thrombin was abolished when hirudin was added simultaneously (2.0 μg/mL of hirudin, 2.6% vs 1.1% in cells treated with 50pM thrombin, P < .001; Figure 5C-D).
Comparable effects were observed in murine podocytes using various concentrations of murine thrombin (data not shown). Therefore, low-dose thrombin mediates protective effects in podocytes, raising the question of whether the nephroprotective effect observed in FVL mice depends on low but sustained thrombin generation.
Anticoagulation with hirudin abolishes the protective effect of FVL
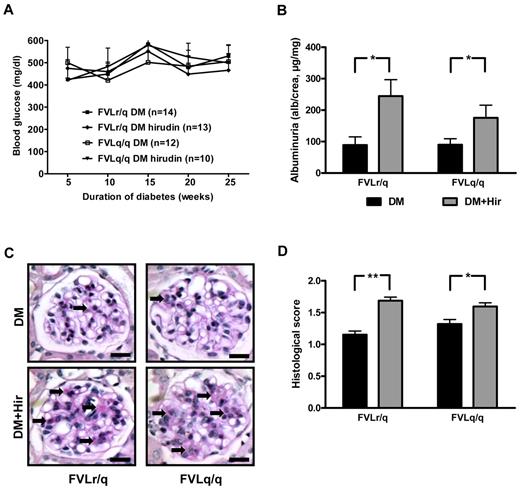
To determine whether the enhanced thrombin generation in FVL-positive mice contributes to the observed amelioration of diabetic nephropathy, we anticoagulated diabetic FVLr/q and FVLq/q mice with the direct thrombin inhibitor hirudin. Anticoagulation with hirudin had no effect on blood glucose levels (Figure 6A), but did reduce TAT levels (26.9 μg/L in diabetic FVLr/q mice and 33.9 μg/L diabetic FVLq/q mice vs 9.0 μg/L and 10.8 μg/L, respectively, in hirudin-treated mice, P = .04 and P = .03, respectively).
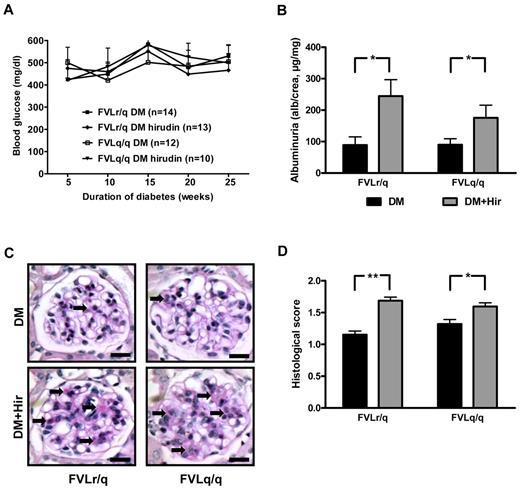
Anticoagulation with hirudin abolishes the nephroprotective effect in diabetic FVL mice. (A) Blood glucose concentrations in diabetic heterozygous (FVLr/q) and diabetic homozygous (FVLq/q) mice without or with hirudin treatment. No significant differences were observed between groups of diabetic mice (n ≥ 10 for each group). (B) Albuminuria in heterozygous (FVLr/q) and homozygous (FVLq/q) mice without (black bars) or with (gray bars) hirudin treatment (n ≥ 10 for each group). (C-D) Representative images of PAS staining (C) and bar graph (D) summarizing histologic scores (n ≥ 50 glomeruli of 7 different mice per group). Scale bar indicates 15 μm (C); means ± SEM are shown. DM indicates diabetic mice (black bars); DM + Hir, diabetic mice with hirudin treatment (gray bars); *P < .05 (t test).
Anticoagulation with hirudin abolishes the nephroprotective effect in diabetic FVL mice. (A) Blood glucose concentrations in diabetic heterozygous (FVLr/q) and diabetic homozygous (FVLq/q) mice without or with hirudin treatment. No significant differences were observed between groups of diabetic mice (n ≥ 10 for each group). (B) Albuminuria in heterozygous (FVLr/q) and homozygous (FVLq/q) mice without (black bars) or with (gray bars) hirudin treatment (n ≥ 10 for each group). (C-D) Representative images of PAS staining (C) and bar graph (D) summarizing histologic scores (n ≥ 50 glomeruli of 7 different mice per group). Scale bar indicates 15 μm (C); means ± SEM are shown. DM indicates diabetic mice (black bars); DM + Hir, diabetic mice with hirudin treatment (gray bars); *P < .05 (t test).
Anticoagulation with hirudin had no significant effect on indices of diabetic nephropathy in diabetic FVLwt mice (data not shown), which is consistent with our previous observation using low–molecular-weight heparin in diabetic wild-type mice.20 Conversely, indices of diabetic nephropathy were significantly increased in hirudin-treated diabetic FVLr/q and FVLq/q mice compared with control diabetic FVLr/q and FVLq/q mice treated with saline injections. Hirudin increased albuminuria to 245.0 μg/mg (vs 90.5 μg/mg, P = .01) in diabetic FVLr/q mice and to 175.4 μg/mg (vs 92.7 μg/mg, P = .04; Figure 6B) in diabetic FVLq/q mice. Likewise, accumulation of extracellular matrix, as determined by PAS staining, was increased (histologic score, 1.7 vs 1.2, P < .001, in diabetic FVLr/q mice; 1.6 vs 1.3, P = .02, in diabetic FVLq/q mice; Figure 6C-D).
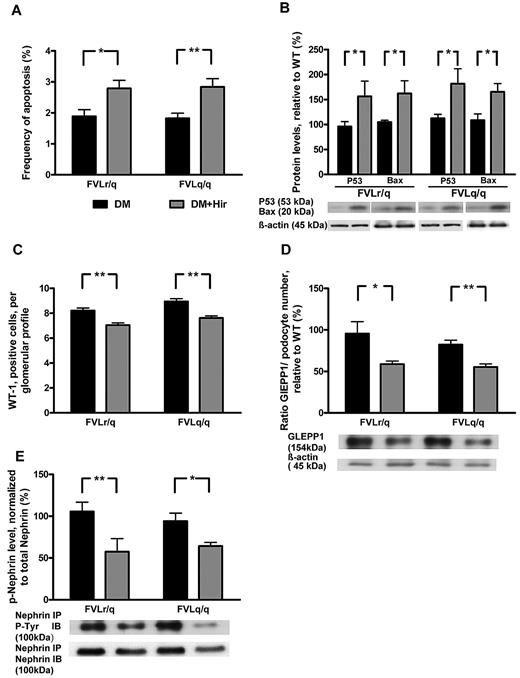
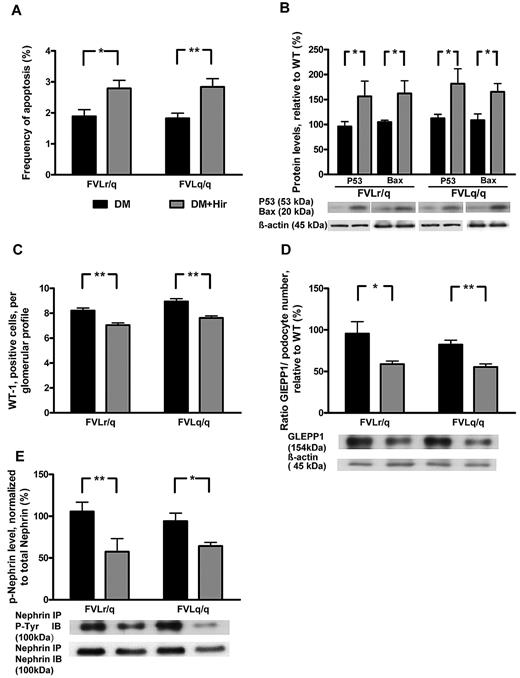
Consistent with thrombin-mediated nephroprotection through inhibition of podocyte apoptosis, the frequency of apoptotic cells in glomeruli (2.8% vs 1.9%, P = .003, in diabetic FVLr/q mice; 2.8% vs 1.8%, P < .001, in diabetic FVLq/q mice; Figure 7A) and the expression of the proapoptotic regulators Bax (162.2% vs 104.9%, P = .04, in diabetic FVLr/q mice; 165.3% vs 108.7%, P = .02, in diabetic FVLq/q mice) and p53 (156.3% vs 95.9%, P = .04, in diabetic FVLr/q mice; 181.6% vs 112.3%, P = .04, in diabetic FVLq/q mice; Figure 7B) increased after treatment with hirudin. The numbers of podocytes was slightly but significantly reduced after hirudin treatment of diabetic FVL-positive mice (7.0 vs 8.2, P < .001, in diabetic FVLr/q mice; 7.6 vs 8.9, P < .001, in diabetic FVLq/q mice; Figure 7C), as was the expression of the podocyte marker GLEPP1 (normalized for podocyte numbers: 58.8% vs 96.2%, P = .04, in diabetic FVLr/q mice; 55.3% vs 81.9%, P = .002, in diabetic FVLq/q mice; Figure 7D). Hirudin not only abolished the protective effect of thrombin in regard to podocyte number, but also reduced the tyrosine phosphorylation of nephrin (57.6% vs 106.1%, P = .009, in diabetic FVLr/q mice; 64.3% vs 94.1%, P = .02, in diabetic FVLq/q mice; Figure 7D), supporting the notion that low but sustained thrombin levels preserve both podocyte number and function in diabetic nephropathy. We conclude that inhibition of thrombin abolishes the protective, FVL-dependent effect in regard to diabetic nephropathy in mice.
Hirudin treatment abolishes podocyte protection in diabetic FVL mice. (A) Bar graph summarizing frequency of apoptotic glomerular cells. (B) Expression of apoptosis regulators in renal cortex tissue samples of diabetic mice without or with hirudin treatment. Bar graph (top) and representative immunoblot (bottom) showing p53 and Bax expression (n ≥ 7 each group). (C) Podocyte number in glomeruli of diabetic mice without or with hirudin treatment as determined by WT-1 immunohistochemical staining. (D) Bar graph (top, data normalized for podocyte numbers) and representative immunoblot (bottom) showing GLEPP1 expression in renal cortex tissue samples of diabetic mice without or with hirudin treatment. Actin immunoblot is shown as loading control (n ≥ 7 each group). (E) Bar graph (top) and representative immunoblots of immunoprecipates (bottom) showing nephrin tyrosine phosphorylation in renal cortex tissue extracts of diabetic mice without or with hirudin treatment (n ≥ 7 each group). DM indicates diabetic mice (black bars); DM + Hir, diabetic mice with hirudin treatment (gray bars); *P < .05; **P < .01 (t test).
Hirudin treatment abolishes podocyte protection in diabetic FVL mice. (A) Bar graph summarizing frequency of apoptotic glomerular cells. (B) Expression of apoptosis regulators in renal cortex tissue samples of diabetic mice without or with hirudin treatment. Bar graph (top) and representative immunoblot (bottom) showing p53 and Bax expression (n ≥ 7 each group). (C) Podocyte number in glomeruli of diabetic mice without or with hirudin treatment as determined by WT-1 immunohistochemical staining. (D) Bar graph (top, data normalized for podocyte numbers) and representative immunoblot (bottom) showing GLEPP1 expression in renal cortex tissue samples of diabetic mice without or with hirudin treatment. Actin immunoblot is shown as loading control (n ≥ 7 each group). (E) Bar graph (top) and representative immunoblots of immunoprecipates (bottom) showing nephrin tyrosine phosphorylation in renal cortex tissue extracts of diabetic mice without or with hirudin treatment (n ≥ 7 each group). DM indicates diabetic mice (black bars); DM + Hir, diabetic mice with hirudin treatment (gray bars); *P < .05; **P < .01 (t test).
Discussion
In the current study, we investigated the role of the FVL mutation in diabetic nephropathy. The presence of the FVL mutation, either heterozygous or homozygous, ameliorated experimental diabetic nephropathy in mice. We also observed lower levels of albuminuria in a retrospective analysis of type 1 and type 2 diabetic patients. Low-dose thrombin prevented glucose-induced podocyte apoptosis in vitro. Furthermore, inhibition of thrombin using the specific and potent thrombin inhibitor hirudin diminished the protective effect observed in diabetic FVL-positive mice with regard to podocyte apoptosis and nephrin tyrosine phosphorylation, indicating that both cell survival and cell function are preserved by low-dose thrombin. Therefore, low but sustained thrombin generation, as observed in FVL carriers, mediates a nephroprotective effect in diabetes mellitus.
Considering the well-established proinflammatory effects of thrombin, the finding that low-dose thrombin mediates nephroprotection appears at first glance to be counterintuitive.27 However, recent studies have established that thrombin can mediate cytoprotective effects in endothelial cells in vitro, suggesting that the hemostatic system has a dual function.26,28 Such a dual function resembles the 2-faced character of reactive oxygen species, also referred to as “redox homeostasis.”29 Given the current and previous results, we propose that the coagulation system has a similar dual function in regulating cellular homeostasis. Thrombin can reduce endothelial cell permeability, expression of adhesion molecules (ICAM-1, VCAM-1, and E-selectin), and adhesion and transendothelial migration of leukocytes through a receptor-dependent (PAR-1) mechanism.26,30 In addition, in TNF-α–stimulated human pulmonary aortic endothelial cells, low-dose thrombin (50pM) was shown to reduce apoptosis.31 Thrombin triggers these cytoprotective effects whether it is present at low doses (∼50pM) or if the endothelial PC receptor is occupied by PC or aPC.26,30,32 The cytoprotective effects of thrombin have also been reported in nonendothelial cells. Thrombin reduces TGF-β–mediated expression of extracellular matrix proteins in renal tubular cells28 and thrombin dose dependently regulates proliferation of rat glioma cells.33 The beneficial effects of low-dose thrombin in endothelial cells and podocytes establish that thrombin can mediate cytoprotection in both cell types constituting the glomerular filtration barrier (endothelial cells and podocytes).
Adding to previous work demonstrating a protective role of thrombin in vitro, we provide in vivo evidence using a murine model for diabetic nephropathy. Consistent with the results from the animal study (a model of type 1 diabetes mellitus), we observed lower average albuminuria in both type 1 and type 2 diabetic patients carrying the FVL mutation. The observed reduction of average albuminuria in type 2 diabetic patients suggests that the protective, FVL-mediated effect observed in the murine type 1 diabetes models is also relevant in the setting of type 2 diabetes mellitus. In contrast to the FVL mutation, which is associated with an increased thrombin-generation potential,34 the PAI-1 4G/5G polymorphism, which increases the risk of thrombotic events without enhancing thrombin formation, is not associated with reduced albuminuria in type 1 or type 2 diabetic patients, as shown in previous studies35-38 and in the current study.
We acknowledge that the observed protection from nephropathy in FVL-positive human diabetic patients requires confirmation in independent studies.39,40 Furthermore, we can currently not exclude species-specific differences in regard to FVL-mediated nephroprotection, considering the differences in the human and murine hemostatic system and podocyte apoptosis for diabetic nephropathy. However, in both humans and mice, the presence of FVL conveys plasma aPC resistance,21 implying a crucial role of low but sustained thrombin generation for nephroprotection in both species. Pending confirmation, the observed association of the FVL mutation, but not the PAI-1 4G/5G polymorphism, with lower average albuminuria is consistent with the proposed nephroprotective function of sustained but low-dose thrombin generation in diabetes mellitus.
The observed nephroprotective effect of low, but sustained thrombin generation observed in diabetic FVL mice and in diabetic patients carrying the FVL mutation extends our previous finding that prothrombotic mutations mediate a partial benefit during atherogenesis.12 Mice carrying the FVL mutation or expressing a mutant thrombomodulin (TMPro/Pro) with severely impaired PC activation and thus reduced anticoagulant function showed larger plaques, but this potentially disadvantageous effect was counterbalanced by increased plaque stability and enhanced vascular remodeling, resulting in a larger vessel diameter.12 In the context of atherosclerotic disease, these beneficial effects are likely counterbalanced at later disease stages by adverse effects during acute vascular events associated with thrombotic vessel occlusion, such as myocardial infarction. It remains to be shown whether hypercoagulability is also associated with both protective and harmful effects in diabetic patients. Detrimental effects at advanced disease stages such as in terminal kidney failure or advanced atherosclerosis can currently not be excluded.
We previously reported a disadvantageous effect of the TMPro mutation, which enhances coagulation activation and impairs PC activation, in experimental diabetic nephropathy.20 In diabetic TMPro/Pro mice, diabetic nephropathy is aggravated despite similar levels of TAT compared with diabetic heterozygous FVL mice, as shown in a previous study20 and in the present study. The key difference between TMPro/Pro and FVL mice is the inability to activate PC in the former, suggesting a dominant-protective effect of aPC in diabetic glomerulopathy. Indeed, whereas elevated levels of aPC completely prevented albuminuria, enhanced thrombin generation in diabetic FVL mice only partially reduced albuminuria and indices of diabetic nephropathy. Consistent with these in vivo data, the extent of endothelial cell apoptosis inhibition is less in low-dose thrombin (50pM)–treated than in aPC (> 10nM)–treated endothelial cells.31 Given the different efficacy of low but sustained thrombin levels and of aPC, we speculate that receptors or intracellular signaling pathways activated by low-dose thrombin or aPC in endothelial cells and podocytes are at least partially different. Furthermore, considering the contrasting phenotypes in diabetic TMPro/Pro and diabetic FVL mice, we can currently not exclude that thrombomodulin itself modulates intracellular signaling. Indeed, a direct function of thrombomodulin in cellular signaling has been demonstrated previously.41,42 Studies delineating the receptors and signaling mechanisms have been initiated.
The complexity of coagulation-dependent cytoprotection is also reflected by the conflicting results obtained in studies evaluating the effect of the FVL mutation in regard to severe inflammation. Initially, Kerlin et al observed enhanced survival of heterozygous FVL carriers in a retrospective analyses of the PROWESS study, a randomized, placebo-controlled study evaluating the efficacy of aPC in septic patients.5,43 Other studies have either confirmed the original observation6,7 or failed to do so.8,9 Results from animal studies added to the controversy. Initially, a survival benefit of heterozygous FVL mice challenged with LPS was reported.5 However, infection with bacteria or cecal ligation and puncture resulted in increased mortality in FVL mice.10,11,44 Interestingly, concomitant antibiotic treatment did result in a survival benefit in heterozygous FVL mice infected with bacteria, which is consistent with the original observation in LPS-treated mice.5,44 This indicates that a survival benefit associated with the FVL mutation may be strongly context dependent. This conclusion is consistent with the proposed dual role of thrombin being either cytoprotective or harmful depending on, for example, its concentration or the concomitant engagement of its coreceptor, endothelial PC receptor.31,32 The pivotal thrombin receptor PAR-1 has a dual function in sepsis, being a vascular-disruptive receptor during early stages and a vascular-protective receptor during later stages of bacterial sepsis in mice.45 The effect of enhanced coagulation activation—or anticoagulation—may also be stage dependent in acute infections or in chronic diseases such as atherosclerosis or diabetic nephropathy. Considering previous and current results, with the emergence of new and specific anticoagulants, well-designed studies addressing their effects on acute and chronic diseases are needed.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
FVL mice were kindly provided by David Ginsburg (University of Michigan, Ann Arbor, MI). We are grateful to Dr Karlhans Endlich (Institute of Anatomy and Cell Biology, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany) for providing mouse podocytes. We thank Marion Künstler for excellent technical support.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (IS 67/2-4), from the Stiftung Pathobiochemie und Molekulare Diagnostik, from the Deutsche Diabetes Stiftung, from the European Association for the Study of Diabetes (to B.I.), and from the Dietmar Hopp Stiftung (to B.I., A.B., and P.P.N.). T.M. has a postdoctoral fellowship from the medical faculty at the University of Heidelberg (Heidelberg, Germany). K.S. has a scholarship from the German Academic Exchange Service (DAAD; Bonn, Germany). T.H. has a scholarship from the China Scholarship Council.
Authorship
Contribution: H.W. and T. M. conducted mouse and in vitro experiments and interpreted the experimental work; T.H., B.H., and I.A.V. conducted mouse and in vitro experiments; S.S. maintained the mouse colony and induced and maintained hyperglycemia in mice; M.K. and K.S. performed blinded histologic analyses; S.M.-K. helped with podocyte culture; G.R. collected patient data and DNA samples; A.B. and V.S supported experimental work; P.P.N. assisted in preparing the manuscript; and B.I. conceptually designed and interpreted the experimental work and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of B.H. is Department of Clinical Chemistry, University of Saarland, Homburg/Saar, Germany. The current affiliation of B.I. is Otto-von-Guericke-University Magdeburg, Department of Clinical Pathology and Pathobiochemistry, Magdeburg, Germany.
Correspondence: Berend Isermann, MD, Otto-von-Guericke-University Magdeburg, Dept of Clinical Pathology and Pathobiochemistry, Leipziger Str 44, 39120 Magdeburg, Germany; e-mail: berend.isermann@med.ovguu.de.
References
Author notes
H.W. and T.M. contributed equally to this study.