In this issue of Blood, Herishanu and colleagues demonstrate that the lymph node is a key site in pathogenesis of chronic lymphocytic leukemia (CLL), where increased signaling through the B-cell receptor (BCR) contributes to proliferation and tumor progression.1 These results have key implications in future laboratory models of CLL, and emphasize the importance of the BCR pathway as a therapeutic target for this disease.
BCR signaling is critical to the development and survival of B cells, and is mediated proximally through phosphorylation of spleen tyrosine kinase (Syk), in both normal and malignant B cells. Using small-molecule inhibitors of Syk, it has recently been appreciated that tonic (non antigen-dependent) BCR signaling contributes to the malignant phenotype in a subset of diffuse large B-cell lymphoma, perhaps through activation of nuclear factor kappa B (NFκB).2,3 The importance of BCR signaling—either antigen dependent or tonic—in maintaining CLL is controversial, but recent studies have demonstrated that Syk is overexpressed in CLL, and that pharmacologic inhibition of Syk results in down-regulation of survival mediators and apoptosis of CLL.4
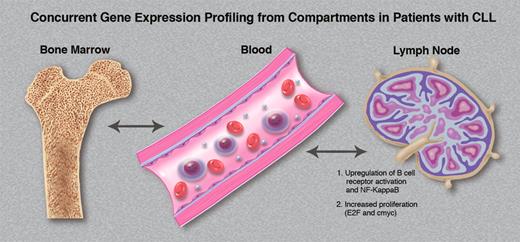
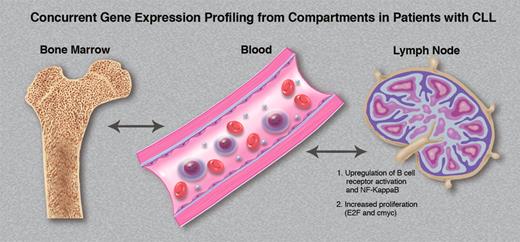
The tissue microenvironment directly affects the tumor biology of CLL cells in vivo. Variable gene expression signatures are observed within the same patients' CLL cells simultaneously obtained from marrow, blood, and lymph node compartments. (Professional illustration by Marie Dauenheimer.)
The tissue microenvironment directly affects the tumor biology of CLL cells in vivo. Variable gene expression signatures are observed within the same patients' CLL cells simultaneously obtained from marrow, blood, and lymph node compartments. (Professional illustration by Marie Dauenheimer.)
The organization of the malignant lymph node microenvironment is critical to prognosis, and almost certainly pathogenesis, in several B-cell malignancies such as Hodgkin lymphoma and follicular lymphoma. In CLL, there is limited data on the nodal microenvironment, and the majority of studies have been restricted to the bone marrow compartment. One important in vitro observation in CLL is that the presence of bone marrow stromal cells can inhibit apoptotic responses to purine analog chemotherapy, through antiapoptotic signals derived from CLL cell–stromal cell contact.5,6 The mechanism for this cross-talk appears to involve chemokines induced by BCR activation.7 Indeed, pharmacologic inhibition of SYK in these model systems can prevent not only BCR signaling-induced CLL survival signals, but also directly block the bone marrow stroma survival signals, potentially abrogating the protective effect of stromal cells in CLL marrow.4
These concepts allow an appreciation for the elegant studies performed by Herishanu and colleagues.1 To further understand the role of the microenvironment in bone marrow in mediating CLL proliferation and survival, in addition to evaluating the importance of the lymph node microenvironment, they purified CLL cells obtained from patients simultaneously from blood, marrow, and lymph node, and performed gene expression profiling. As shown in the figure, the tissue microenvironment directly affects the tumor biology of CLL cells in vivo, with variable gene expression signatures observed within the same patient's CLL cells from the various compartments. Increased BCR signaling (and resultant Syk phosphorylation) was observed in lymph node–derived CLL cells. NFκB was activated preferentially in nodal cells through the canonical pathway, resulting in proliferation and c-myc activation within the microenvironment. The tumor proliferation observed within the lymph node correlated with disease progression in the patient, suggesting that the microenvironment within the lymph node may be a key determinant with regard to disease course and outcome.
These fascinating results provide a framework for an increased understanding of the pathogenesis of CLL, emphasizing the microenvironment as vital player. Based upon these results, in vitro drug studies from blood-derived cells are of limited value in predicting responses to therapy, and future models of CLL must account for the complicated cross-talk between CLL and stroma present within nodal tissue. Moreover, a recent clinical trial in CLL targeting Syk had a high response rate, and observed rapid lymph node responses and subsequent transient lymphocytosis.8 Similar findings have been suggested in preliminary results of trials targeting other elements of the BCR pathway, including phosphatidylinositol 3-kinase, and Bruton tyrosine kinase. We now understand the importance of BCR signaling in maintaining the CLL nodal malignant microenvironment, which sheds light on these clinical observations. Given the importance of BCR signaling in maintaining the CLL nodal malignant microenvironment, we have even more reason to pursue this pathway as a rational therapeutic target for this disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health