Abstract
Natural killer (NK) cells are granular lymphocytes that are generated from hematopoietic stem cells and play vital roles in the innate immune response against tumors and viral infection. Generation of NK cells is known to require several cytokines, including interleukin-15 (IL-15) and Fms-like tyrosine kinase 3 ligand, but not IL-2 or IL-7. Here we investigated the in vivo role of CXC chemokine ligand-12 (CXCL12) and its primary receptor CXCR4 in NK-cell development. The numbers of NK cells appeared normal in embryos lacking CXCL12 or CXCR4; however, the numbers of functional NK cells were severely reduced in the bone marrow, spleen, and peripheral blood from adult CXCR4 conditionally deficient mice compared with control animals, probably resulting from cell-intrinsic CXCR4 deficiency. In culture, CXCL12 enhanced the generation of NK cells from lymphoid-primed multipotent progenitors and immature NK cells. In the bone marrow, expression of IL-15 mRNA was considerably higher in CXCL12-abundant reticular (CAR) cells than in other marrow cells, and most NK cells were in contact with the processes of CAR cells. Thus, CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adults, and CAR cells might function as a niche for NK cells in bone marrow.
Introduction
Natural killer (NK) cells are large, granular lymphocytes that have an important role in innate immune response against tumors, viral infection, and graft rejection.1,2 They also elicit adaptive immune responses by producing cytokines, such as interferon-γ (IFN-γ), tumor-necrosis factor-α, and granulocyte-macrophage colony-stimulating factor and chemokines, such as CC chemokine ligand-3 (CCL3), CCL4, and CCL5, as proinflammatory mediators.2
NK cells are generated from hematopoietic stem cells (HSCs) in the fetal liver and adult bone marrow. Bipotent T-cell and NK-cell progenitors (p-T/NKs) have been identified in the fetal liver3 and fetal thymus,4,5 and the earliest committed NK-cell precursors (NKPs), which are characterized by the expression of CD122 (interleukin-2 receptorβ [IL-2Rβ]),5 and mature NK cells (mNKs), which express NK-cell markers NK1.1 and DX5,6 have been detected in the fetal thymus. In adult mice, HSCs give rise to lymphoid-primed multipotent progenitors (LMPPs)7 and common lymphoid progenitors (CLPs),8,9 which have the potential to generate NK cells as well as T, B, and dendritic cells (DCs). As in the fetal thymus, Lin−NK1.1−DX5−CD122+ cells have been reported to be the earliest committed NKPs in the marrow.10 The development of NKPs to immature NK cells (iNKs) is accompanied by the expression of NK1.1, and developing iNKs acquire the expressions of DX5, Mac-1, and Ly49 receptors, including inhibitory or activating NK cell receptors, in an orderly fashion and differentiate into mNKs.2,11
Generation of NK cells is regulated by several cytokines produced by microenvironments in the fetal liver and/or bone marrow. In mice lacking IL-15 or its receptors, including IL-15 receptor α (IL-15Rα), IL-2Rβ, and IL-2Rγ, the numbers of NKPs are relatively normal, but the number and cytotoxic activity of NK cells are severely reduced compared with control animals.12-15 In addition, Fms-like tyrosine kinase 3 ligand (Flt3L)–deficient mice have a reduction in the number and cytotoxic activity of NK cells in the bone marrow.16 In contrast, IL-2 or IL-7 has been shown to be dispensable for NK-cell development because the numbers of NK cells are normal in mice lacking IL-2,17,18 IL-7,18,19 or IL-7Rα.20 The role of stem cell factor (SCF) and its receptor c-kit in NK-cell development remains a controversial issue. Although viable W/W mice lacking c-kit, termed Vickid mice, show normal NK-cell development,21 chimeric mice reconstituted with fetal liver cells from W/W mice had a reduction in the number and function of NK cells.22
CXC chemokine ligand-12 (CXCL12; also known as stromal cell–derived factor-1 and pre-B-cell-growth-stimulating factor) is a member of the chemokines, a large family of structurally related chemoattractive cytokines, and its primary physiologic receptor is CXCR4, a hepta-helical receptor coupled to heterotrimeric G proteins, which also functions as an entry receptor for HIV-1.23 Previous studies using mice lacking CXCL12 or CXCR4 have revealed that CXCL12-CXCR4 signaling is essential for the colonization of bone marrow by hematopoietic cells, including HSCs during ontogeny, maintenance of HSCs in the adult bone marrow, and development of B cells and plasmacytoid DCs (pDCs).23-30 A recent study has shown that CXCL12 induces NK-cell migration and that administration of CXCR4 antagonist AMD3100 induced the reduction of NK cells in the bone marrow and their increase in spleen and peripheral blood, suggesting that CXCL12 regulates the retention of NK cells in the bone marrow31 ; however, the in vivo role of the CXCL12-CXCR4 signaling in NK-cell development remains unclear.
In this study, we have shown that CXCL12-CXCR4 chemokine signaling is dispensable for NK-cell development during ontogeny but plays a critical role in the generation of NK cells in adults.
Methods
Mice
CXCR4null/null mice,25 CXCL12-GFP knock-in mice,28 and CXCR4flox(f)/null mice32 were maintained on a C57BL/6-Ly5.2+/Ly5.1− background. CXCR4wt/null mice were intercrossed to generate CXCR4−/− embryo. Fetal thymuses were obtained from day 15.5 or 17.5 embryo, and the genotypes were determined by polymerase chain reaction (PCR) analysis. CXCR4f/null mice were crossed with MxCre transgenic mice to generate Cre-mediated CXCR4 conditionally deficient mice as described.29 For removal of floxed CXCR4 allele, the MxCre/CXCR4f/null mice were injected subcutaneously with polyinosinic acid-polycytidylic acid (pIpC; 20 μg/g body weight; GE Healthcare) 8 times every other day from 3 days after birth, and were analyzed at 12 to 15 weeks after final pIpC treatment. All animal experimentation was conducted in accordance with the guidelines of the Institute for Frontier Medical Sciences, Kyoto University.
Antibodies
The following antibodies were used for flow cytometry: anti-CD3ϵ (145-2C11), anti-CD4 (RM4-5), anti-CD8a (53-6.7), anti-NK1.1 (PK136), anti-CD49b (DX5), anti-CD122 (TMβ1), anti-CD44 (IM7), anti-CD25 (7D4), anti-CD11b (M1/70), anti-CD27 (LG.3A10), anti-KLRG1 (2F1), anti-Ly49A (A1), anti-Ly49C/I (5E6), anti-Ly49D (4E5), anti-Ly49G2 (LGL-1), anti-CD94 (18d3), anti–IFN-γ (XMG1.2), anti–c-kit (2B8), anti–Sca-1 (D7), anti-Flt3 (A2F10), anti-CD45R (RA3–6B2), anti–Gr-1 (RB6–8C5), anti–TER-119 (TER-119), anti-CD19 (1D3), anti-Ly5.2 (104), anti-Ly5.1 (A20), CD31 (MEC13.3), CD11c (HL3), and major histocompatibility complex (MHC) class II (M5/114.15.2). All of these antibodies were directly conjugated to fluorescein isothiocyanate, phycoerythrin, phycoerythrin-Cy7, allophycocyanin, allophycocyanin-Cy7, Pacific blue, or biotin and were purchased from BD Biosciences or eBioscience. Biotinylated antibodies were detected with phycoerythrin-Cy7 (BD Biosciences) or Pacific blue (Invitrogen)–conjugated streptavidin.
Flow cytometric analysis and cell sorting
Single-cell suspensions were prepared from freshly isolated fetal thymus, adult bone marrow, spleen, lung, and peripheral blood. Cells were stained with monoclonal antibodies against cell surface markers and its secondary reagents. Dead cells were excluded by propidium iodide staining. All flow cytometric analysis and cell sorting were performed with FACSAria (BD Biosciences). For intracellular IFN-γ staining, single-cell suspensions from spleen were incubated at 1 × 106 cells/mL in 48-well plate in RPMI 1640 supplemented with 10% fetal calf serum (FCS; SAFC Biosciences), 50μM 2-mercaptoethanol, 50 μg/mL streptomycin, 75 μg/mL penicillin, 1 ng/mL IL-12 (PeproTech), 10 ng/mL IL-18 (MBL). After 2 hours of incubation, 10 μg/mL brefeldin A (Sigma-Aldrich) was added and incubated for an additional 4 hours. Fixation and permeabilization were performed with Cytofix/Cytoperm solution (BD Biosciences), followed by intracellular staining with allophycocyanin-conjugated anti–IFN-γ. Cell-cycle analysis was performed as described in our previous publication.29 For the apoptosis assay, annexin V–FITC Apoptosis Detection Kit I (BD Biosciences) was used according to the manufacturer's instruction. For sorting the nonhematopoietic cell populations, cells in bone marrow fraction were obtained from femurs and tibiae by flushing and collagenase (Sigma-Aldrich) digestion.
Migration assay
Chemotactic migration assays with or without CXCL12 (200 μg/mL) were performed in 5-μm pore Transwell inset (Corning Life Sciences). Cells from bone marrow and spleen (5 × 105) were loaded on the upper well. After 90 minutes, the migrated cells from the lower well were harvested and analyzed by FACSAria.
Competitive repopulation assay
The competitive repopulation assay was performed as previously described.29 Briefly, unfractionated 2 × 106 bone marrow cells from Ly5.2+/Ly5.1− MxCre-CXCR4f/wt or MxCre-CXCR4f/null mice were mixed with 1 × 106 bone marrow cells from C57BL/6-Ly5.2−/Ly5.1+ mice as competitor cells and were transferred into lethally irradiated (9 Gy) C57BL/6-Ly5.2−/Ly5.1+ recipient mice. Mice were treated with pIpC at 12 weeks after transfer and were analyzed by flow cytometry at 15 weeks after final pIpC treatment.
Cytotoxicity assay
Lactate dehydrogenase (LDH) release assay was used to measure NK lytic activity against the NK-sensitive target YAC-1 cells in vitro. A total of 3 × 103 target cells were incubated with serially diluted effector cells in 0.1 mL of complete medium (RPMI 1640 supplemented with 2% FCS, 50μM 2-mercaptoethanol, 50 μg/mL streptomycin, 75 μg/mL penicillin) for 4 hours. Effector cells were bone marrow DX5+ cells from 17-week-old mice that were enriched by positive selection with MACS DX5 microbeads (Miltenyi Biotec) or CD3−NK1.1+ cells that were sorted (> 99%) from bone marrow and spleen of 12-week-old mice by flow cytometry. LDH released on cell lysis was measured by Non-Radioactive Cytotoxicity Assay (CytoTox96; Promega) according to the manufacturer's instructions. The percentage of specific lysis was calculated as follows: 100 × (experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous).
In vitro culture
Lin−Sca-1+c-kit+Flt3+ cells (LMPPs) (100 cells/well) and CD3−NK1.1+DX5− iNK cells (2000-3000 cells/well) sorted from bone marrow were cultured in 96-well U-bottomed plates in 0.1 mL of complete medium (RPMI 1640 with 10% FCS, 50μM 2-mercaptoethanol, 50 μg/mL streptomycin, 75 μg/mL penicillin, sodium pyruvate, and nonessential amino acids). For culture initiated with LMPPs, Flt3L, SCF (R&D Systems), IL-15 (PeproTech), and CXCL12 were used at 10 ng/mL, 20 ng/mL, 20 ng/mL, and 1 μg/mL, respectively. The numbers of CD3−NK1.1+ NK cells were measured by flow cytometry on day 14 of culture. For culture initiated with iNKs, IL-15 and CXCL12 were used at 10 ng/mL and 1 μg/mL, respectively. The numbers of CD3−NK1.1+DX5+ mNK cells were measured by flow cytometry on day 3 of culture.
Quantitative RT-PCR analysis
Total RNA was extracted from sorted cells with Isogen (Nippon Gene). After DNaseI (Invitrogen) treatment, reverse transcription was performed using SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to the manufacturer's instructions. Quantitative PCR analysis was performed with a Step One Plus (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems). Values for each gene were normalized to the relative quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA in each sample. The sequences of the primers used for the PCR reaction are as follows: GAPDH F, 5′-TCATGAGCCCTTCCACAATG-3′; GAPDH R, 5′-GGTGTGAACCACGAGAAATATGAC-3′; Perforin F, 5′-AAGGTAGCCAATTTTGCAGC-3′; Perforin R, 5′-GGTTTTTGTACCAGGCGAAA-3′; E4BP4 F, 5′-CGGAAGTTGCATCTCAGTCA-3′; E4BP4 R, 5′-GCAAAGCTCTCCAACTCCAC-3′; CXCR4 F, 5′-TAGGATCTTCCTGCCCACCAT-3; CXCR4 R, 5′-TGACCAGGATCACCAATCCA-3′; IL-15 F, 5′-ACATCCATCTCGTGCTACTTGT-3′; IL-15 R, 5′-GCTCGCATGCAGTCAGGAC-3′; IL-15Rα F, 5′-GCTTTCCTGGCCTGGTACATC-3′; and IL-15Rα R, 5′-CTGCTGGCCCTCACAGTCAT-3′.
Single-cell RT-PCR analysis
Single cells were sorted into reverse transcription buffer containing 1 U/μL RNase Inhibitor (Toyobo) and 0.4% Nonidet P-40 (Nacalai Tesque) using flow cytometry. Cell lysates were reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Toyobo) and gene-specific reverse primers. PCR was subsequently performed by the addition of premixed hot-start PCR enzymes and buffers (AmpliTaq Gold, Applied Biosystems) containing the gene-specific forward and reverse primers designed to span introns to exclude genomic products. PCR was carried out in one round with 50 amplification cycles. A total of 100 cells per mouse were analyzed.
Immunohistochemical analysis
Immunostaining was performed as previously described.29 In brief, 7-μm-thick 4% paraformaldehyde-fixed cryostat sections were first blocked with 5% FCS/phosphate-buffered saline and stained with goat anti–mouse NKp46 (R&D Systems), followed by secondary donkey anti–goat IgG-Cy3 (Jackson ImmunoResearch Laboratories). Sections were visualized with a LSM 510 META (Carl Zeiss) using a 40×/1.3 NA oil-immersion objective lens (Carl Zeiss). All acquired images were processed with the LSM Image Browser (Carl Zeiss).
Statistical analysis
Data were expressed as mean plus or minus SD. The statistical significances between groups were evaluated using the 2-tailed Student t test.
Results
CXCR4-CXCL12 signaling is required for NK-cell development in adults but not in embryos
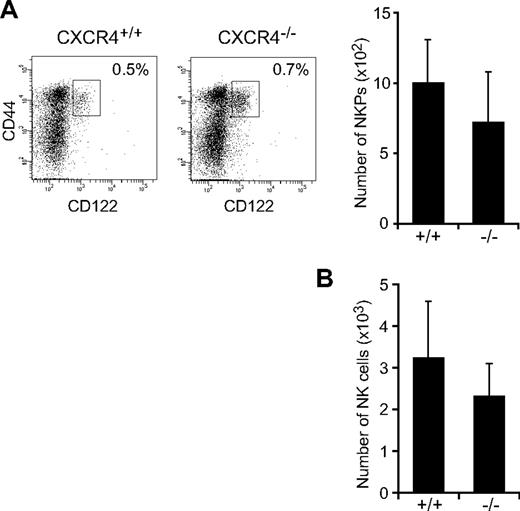
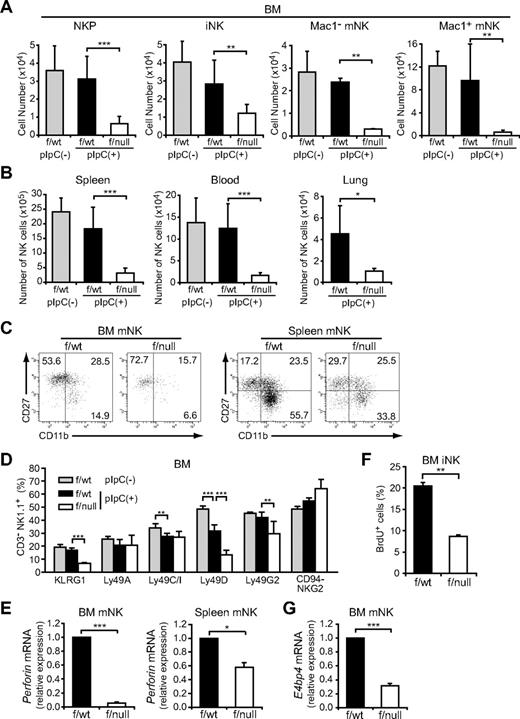
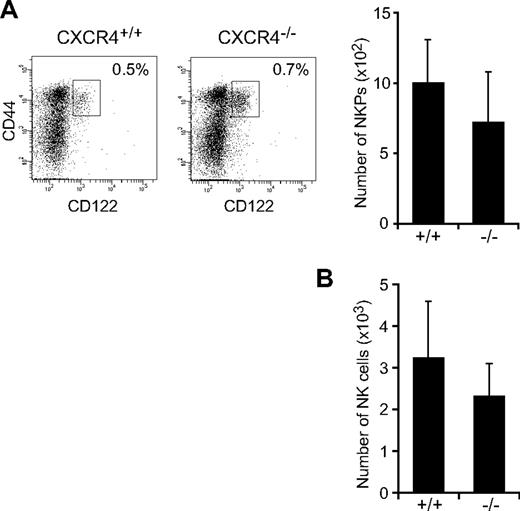
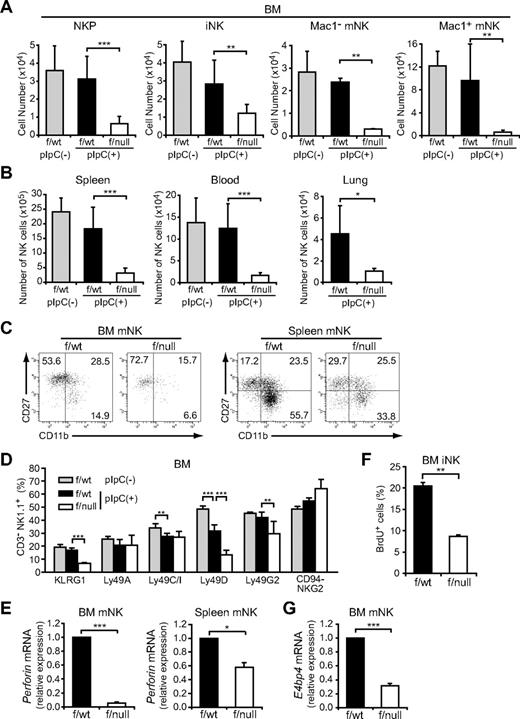
To determine the in vivo role of CXCL12-CXCR4 signaling in NK-cell development, we first analyzed the numbers of NK-cell progenitors in CXCR4null/null embryos. A previous study showed that the numbers of p-T/NKs in the fetal liver were unaffected in CXCL12-deficient embryos.27 Flow cytometric analysis of fetal thymocytes revealed no significant differences in the numbers of Lin−NK1.1−CD44+CD25−CD122+ NKPs (Figure 1A) and CD3−NK1.1+DX5+ mNKs (Figure 1B) between wild-type and CXCR4null/null embryos, suggesting that the CXCL12-CXCR4 signaling is dispensable for NK-cell development in embryos. Because conventional CXCR4null/null mice die in the embryonic stage, we next analyzed the bone marrow of MxCre/CXCR4f/null mice, in which Cre was expressed after the induction of type I IFN by the administration of pIpC to inactivate the CXCR4 gene in the adult animals.29 In CXCR4 conditionally deficient mice, the numbers of CLPs in the bone marrow were severely reduced compared with control animals.30 Flow cytometric analysis revealed that, although the numbers of T-cell precursors, including CD4−CD8− (DN) and CD4+CD8+ (DP) cells in the thymus, were relatively unimpaired (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), the numbers of NK-cell precursors, including Lin−NK1.1−DX5−CD122+ NKPs and CD3−NK1.1+DX5− iNKs, were reduced in the bone marrow of pIpC-treated MxCre/CXCR4f/null mice compared with pIpC-treated or untreated MxCre/CXCR4f/wt mice (Figure 2A; supplemental Figure 2). In addition, the frequencies and numbers of CD3−NK1.1+DX5+ mNKs, including Mac-1− and Mac-1+ subsets in the bone marrow (Figure 2A) and mNKs in the spleen, lung, and peripheral blood (Figure 2B; supplemental Figure 2; and data not shown), were more severely reduced in pIpC-treated MxCre/CXCR4f/null mice compared with control animals. Of note, the numbers of CD27+Mac-1+ (KLRG1−) subset of mNKs, which are thought to display a greater effector function,33 and KLRG1+ end-stage mNKs34 were severely reduced in bone marrow and spleen of pIpC-treated MxCre/CXCR4f/null mice (Figure 2C-D; and data not shown). In the mutants, expression of CXCR4 mRNA and migratory response to CXCL12 observed in NK cells from CXCR4 deleted mice was severely reduced as expected (supplemental Figure 3). Most Ly49 NK cell receptors exhibited normal expression levels, although inhibitory receptor Ly49G2 was abnormally expressed in CD3−NK1.1+ NK cells from the bone marrow of pIpC-treated MxCre/CXCR4f/null mice (Figure 2D). Perforin is a key effector molecule for NK cell–mediated cytolysis. Quantitative, real-time PCR with reverse transcription (RT) analysis revealed that the expression of Perforin was reduced in mNKs from the bone marrow and spleen of pIpC-treated MxCre/CXCR4f/null mice compared with pIpC-treated MxCre/CXCR4f/wt mice (Figure 2E).
Normal cell numbers of NK cells in CXCR4-deficient fetal thymus. Flow cytometric analysis of NKPs and NK cells in CXCR4null/null fetal thymus. (A) Fluorescence staining profile of Lin− (TER119−Mac-1−Gr-1−B220−CD3−CD4−CD8−) NK1.1−CD25− cells (left) and the numbers of Lin−NK1.1−CD44+CD25−CD122+ NKPs (right) in E15.5 fetal thymus. (B) The numbers of CD3−NK1.1+DX5+ mNKs in E17.5 fetal thymus; n = 4.
Normal cell numbers of NK cells in CXCR4-deficient fetal thymus. Flow cytometric analysis of NKPs and NK cells in CXCR4null/null fetal thymus. (A) Fluorescence staining profile of Lin− (TER119−Mac-1−Gr-1−B220−CD3−CD4−CD8−) NK1.1−CD25− cells (left) and the numbers of Lin−NK1.1−CD44+CD25−CD122+ NKPs (right) in E15.5 fetal thymus. (B) The numbers of CD3−NK1.1+DX5+ mNKs in E17.5 fetal thymus; n = 4.
The severe developmental defect of NK cells in CXCR4 conditionally deficient mice. (A-D) Flow cytometric analysis of the numbers of Lin− (TER119−Mac-1−Gr-1− CD3−CD4−CD8−) NK1.1−DX5−CD122+ NKPs, CD3−NK1.1+DX5− iNKs, and Mac-1− and Mac-1+ subsets of CD3−NK1.1+DX5+ mNKs in the bone marrow (2 femurs and tibiae) (A), and CD3−NK1.1+DX5+ mNKs in spleen, lung, and peripheral blood (B) from untreated MxCre/CXCR4f/wt, pIpC-treated MxCre/CXCR4f/wt, or MxCre/CXCR4f/null mice; n = 4. (C) Immunofluorescent profiles of NK cells. Gated CD3−NK1.1+DX5+ are analyzed for the expression of Mac-1 and CD27. (D) Flow cytometric analysis of the frequencies of bone marrow CD3−NK1.1+ NK cells expressing KLRG1, Ly49A, C/I, D, G2, or CD94/NKG2; n = 4. (E,G) Quantitative RT-PCR analysis of mRNA expression of Perforin in CD3−NK1.1+DX5+ mNKs in the bone marrow and spleen (E) and E4BP4 in mNKs in the bone marrow (G) from pIpC-treated MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice. Results are expressed as fold difference compared with the levels found in samples from MxCre/CXCR4f/wt mice (GAPDH normalization); n = 3. (F) BrdU was administered over a 3-day period. The frequencies of CD3−NK1.1+DX5− iNKs incorporated BrdU over this period were analyzed by flow cytometry; n = 3. *P < .05. **P < .01. ***P < .001.
The severe developmental defect of NK cells in CXCR4 conditionally deficient mice. (A-D) Flow cytometric analysis of the numbers of Lin− (TER119−Mac-1−Gr-1− CD3−CD4−CD8−) NK1.1−DX5−CD122+ NKPs, CD3−NK1.1+DX5− iNKs, and Mac-1− and Mac-1+ subsets of CD3−NK1.1+DX5+ mNKs in the bone marrow (2 femurs and tibiae) (A), and CD3−NK1.1+DX5+ mNKs in spleen, lung, and peripheral blood (B) from untreated MxCre/CXCR4f/wt, pIpC-treated MxCre/CXCR4f/wt, or MxCre/CXCR4f/null mice; n = 4. (C) Immunofluorescent profiles of NK cells. Gated CD3−NK1.1+DX5+ are analyzed for the expression of Mac-1 and CD27. (D) Flow cytometric analysis of the frequencies of bone marrow CD3−NK1.1+ NK cells expressing KLRG1, Ly49A, C/I, D, G2, or CD94/NKG2; n = 4. (E,G) Quantitative RT-PCR analysis of mRNA expression of Perforin in CD3−NK1.1+DX5+ mNKs in the bone marrow and spleen (E) and E4BP4 in mNKs in the bone marrow (G) from pIpC-treated MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice. Results are expressed as fold difference compared with the levels found in samples from MxCre/CXCR4f/wt mice (GAPDH normalization); n = 3. (F) BrdU was administered over a 3-day period. The frequencies of CD3−NK1.1+DX5− iNKs incorporated BrdU over this period were analyzed by flow cytometry; n = 3. *P < .05. **P < .01. ***P < .001.
The effect of CXCR4 depletion on the proliferation and survival of NK cells was examined using in vivo assays. We determined the proportion of NK cells from pIpC-treated MxCre/CXCR4f/null mice that incorporate bromodeoxyuridine (BrdU) over a 3-day period and used annexin V to stain these cells. The frequencies of iNK-incorporated BrdU over this period were decreased (Figure 2F), although there is no significant increase in annexin V–positive apoptotic NK cells in the absence of CXCR4 (supplemental Figure 4), suggesting that CXCL12 promotes proliferation of iNKs.
A recent study has shown that transcription factor E4BP4 is preferentially expressed in NK cells as well as NKT cells and is essential for NK-cell development, but not for NKT-cell development.35 Quantitative RT-PCR analysis revealed that E4BP4 mRNA expression was reduced in mNKs from the bone marrow of pIpC-treated MxCre/CXCR4f/null mice compared with pIpC-treated MxCre/CXCR4f/wt mice (Figure 2G). This supports the idea that CXCR4 is essential for development of NK lineage and raises the possibility that CXCL12-CXCR4 signaling is involved in E4BP4 up-regulation in NK lineage cells.
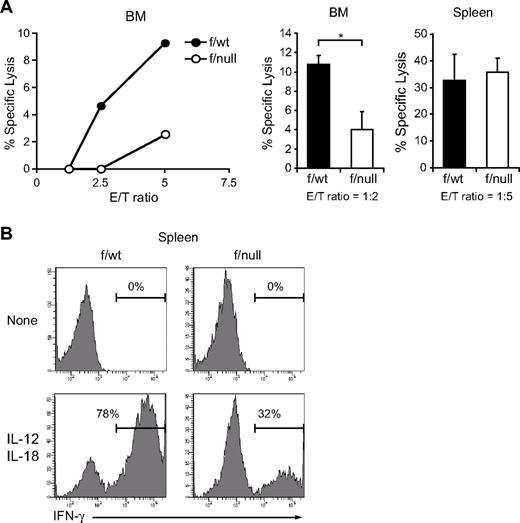
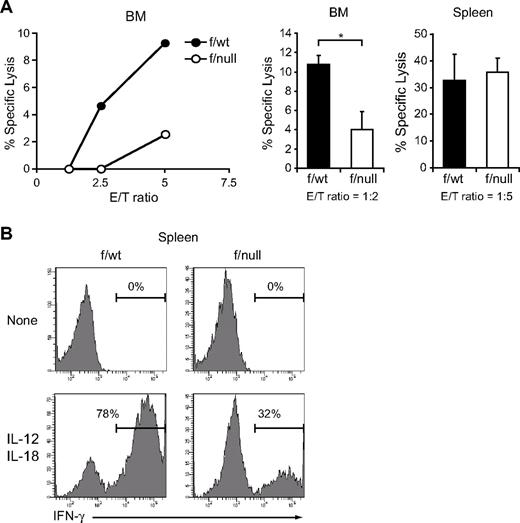
Next, to determine the numbers of functional NK cells, we assessed the capacity of freshly isolated NK cells to lyse YAC-1 targets in a LDH release assay and to produce IFN-γ in response to IL-12 and IL-18. In pIpC-treated MxCre/CXCR4f/null mice, NK cells from bone marrow showed reduced cytotoxicity against YAC-1 targets (Figure 3A), and NK cells from bone marrow and spleen contained decreased numbers of IFN-γ–producing cells (Figure 3B; and data not shown) compared with those from pIpC-treated MxCre/CXCR4f/wt mice. Considering the reduction in the number of CD3−NK1.1+ NK cells in CXCR4 conditionally deficient mice, these results indicate that the numbers of functional NK cells were severely reduced in the absence of CXCR4.
Decreased functional NK cells in CXCR4 conditionally deficient mice. (A) In vitro cytotoxicity of NK cells in the bone marrow (left and middle) or spleen (right) from pIpC-treated MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice. LDH release assay was used to measure NK lytic activity against the NK cell-sensitive YAC-1 target cells. DX5+ cells purified by magnetic-activated cell sorting (left) and sorted CD3−NK1.1+ NK cells (middle and right) were used as effector cells and were incubated with YAC-1 target cells at the indicated effector-to-target cell ratios (E/T) for 4 hours. Data are representative of 3 experiments; n = 3. *P < .05. (B) Flow cytometric analysis of in vitro IFN-γ production by NK cells in the spleen from pIpC-treated MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice. Splenocytes were stimulated with IL-12 and IL-18 for 6 hours. IFN-γ production was measured in CD3−NK1.1+ NK cells by intracellular staining.
Decreased functional NK cells in CXCR4 conditionally deficient mice. (A) In vitro cytotoxicity of NK cells in the bone marrow (left and middle) or spleen (right) from pIpC-treated MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice. LDH release assay was used to measure NK lytic activity against the NK cell-sensitive YAC-1 target cells. DX5+ cells purified by magnetic-activated cell sorting (left) and sorted CD3−NK1.1+ NK cells (middle and right) were used as effector cells and were incubated with YAC-1 target cells at the indicated effector-to-target cell ratios (E/T) for 4 hours. Data are representative of 3 experiments; n = 3. *P < .05. (B) Flow cytometric analysis of in vitro IFN-γ production by NK cells in the spleen from pIpC-treated MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice. Splenocytes were stimulated with IL-12 and IL-18 for 6 hours. IFN-γ production was measured in CD3−NK1.1+ NK cells by intracellular staining.
CXCR4 is required for NK-cell development in a cell-intrinsic manner
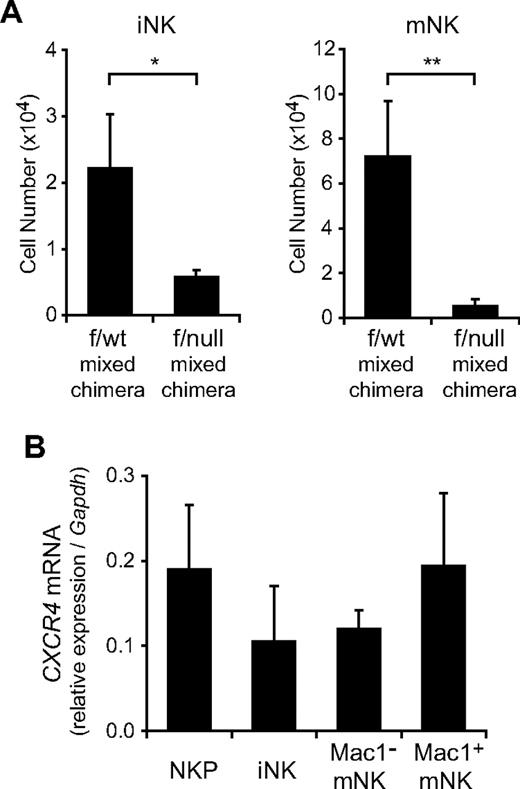
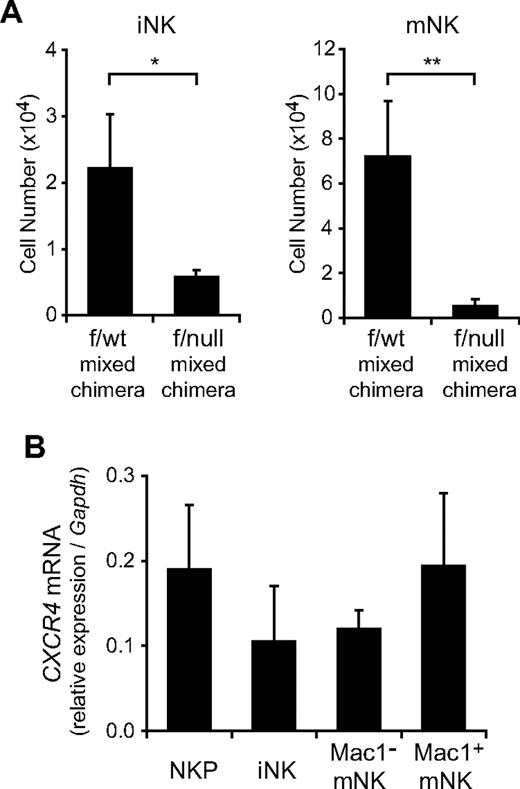
Our result that CXCR4 is essential for NK-cell development raised the question of whether the defect of NK-cell development in the absence of CXCR4 was attributed to an intrinsic NK lineage cell defect or to a microenvironmental defect. To address this issue, we injected 2 × 106 bone marrow cells from Ly5.2+/Ly5.1− MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice with or without 1 × 106 Ly5.2−/Ly5.1+ wild-type bone marrow hematopoietic cells into lethally irradiated wild-type Ly5.2−/Ly5.1+ mice. When chimeric mice exhibited long-term multilineage reconstitution by donor HSCs at 12 weeks after transfer, mice were treated with pIpC to induce excision of the floxed allele. At 15 weeks after final pIpC treatment, flow cytometric analysis revealed that the numbers of donor-derived NKPs, iNKs, and mNKs were severely reduced in the bone marrow from mutant chimeras with or without wild-type hematopoietic cells compared with control chimeras (Figure 4A; and data not shown). These results indicate that the defect of NK-cell development in the absence of CXCR4 is attributed to a cell-intrinsic CXCR4 deficiency. Consistent with this, CXCR4 mRNA is expressed in NKPs, iNKs, and mNKs from wild-type bone marrow (Figure 4B). Differences in the expression levels of CXCR4 mRNA between NKPs, iNKs, and mNKs are not statistically significant, although previous reports have shown a progressive reduction of cell surface expression level during maturation.31,33 Cell surface expression of CXCR4 in mNK cells might be down-regulated by ligand-induced receptor internalization.
The developmental defect of NK cells in CXCR4 conditionally deficient mice is cell-intrinsic. Ly5.2+/Ly5.1− bone marrow cells from MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice were mixed with Ly5.2−/Ly5.1+ wild-type bone marrow cells and transplanted into lethally irradiated normal Ly5.2−/Ly5.1+ wild-type recipients. At 12 weeks after transplantation, mice were treated with pIpC. At 15 week after final pIpC treatment, recipient mice were analyzed by flow cytometry. (A) The numbers of donor-derived Ly5.2+CD3−NK1.1+DX5− iNKs and Ly5.2+CD3−NK1.1+DX5+ mNKs; n = 4. *P < .05. **P < .01. (B) Quantitative RT-PCR analysis of mRNA expression of CXCR4 in NKPs, CD3−NK1.1+DX5− iNKs, and CD3−NK1.1+DX5+ mNKs in the bone marrow from wild-type mice. Data are normalized to GAPDH levels; n = 3.
The developmental defect of NK cells in CXCR4 conditionally deficient mice is cell-intrinsic. Ly5.2+/Ly5.1− bone marrow cells from MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice were mixed with Ly5.2−/Ly5.1+ wild-type bone marrow cells and transplanted into lethally irradiated normal Ly5.2−/Ly5.1+ wild-type recipients. At 12 weeks after transplantation, mice were treated with pIpC. At 15 week after final pIpC treatment, recipient mice were analyzed by flow cytometry. (A) The numbers of donor-derived Ly5.2+CD3−NK1.1+DX5− iNKs and Ly5.2+CD3−NK1.1+DX5+ mNKs; n = 4. *P < .05. **P < .01. (B) Quantitative RT-PCR analysis of mRNA expression of CXCR4 in NKPs, CD3−NK1.1+DX5− iNKs, and CD3−NK1.1+DX5+ mNKs in the bone marrow from wild-type mice. Data are normalized to GAPDH levels; n = 3.
In vitro effect of CXCL12 on generation of NK cells from LMPPs and iNKs
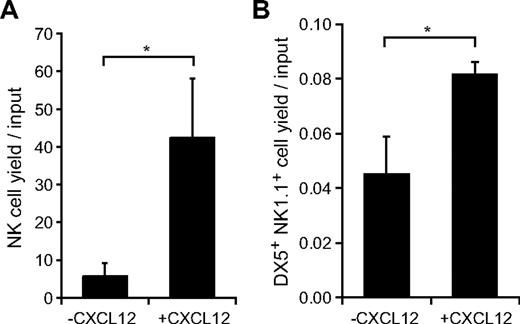
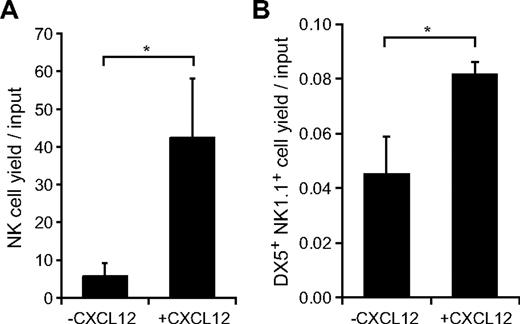
It has been reported previously that Lin− Sca-1+c-kit+ (LSK) Flt3hi LMPPs are the first lymphoid-primed progenitors that give rise to NK cells in vitro and in vivo.7 We examined the in vitro activities of CXCL12 in the generation of NK cells from LMPPs and the generation of mNKs from iNKs. LSK Flt3hi and CD3−NK1.1+DX5− cells were sorted from the bone marrow of wild-type mice and cultured in medium containing Flt3L, SCF, and IL-15, which have been reported to be involved in NK-cell development in vivo,12-16,22 in culture initiated with LMPPs or IL-15 in culture initiated with iNKs in the absence or presence of CXCL12. The numbers of NK cells were markedly increased with the addition of CXCL12 after 14-day culture initiated with LMPPs (Figure 5A). In addition, the numbers of CD3−NK1.1+DX5+ mNKs were increased but to a lesser extent with the addition of CXCL12 after 3-day culture initiated with iNKs (Figure 5B). These results support the idea that CXCL12 is involved in the generation of NK cells from LMPPs and iNKs.
In vitro effects of CXCL12 on generation of NK cells from LMPPs or iNKs. (A) Sorted Lin−Sca-1+c-kit+ (LSK) Flt3hi LMPPs were cultured in medium containing Flt3L, SCF, and IL-15 in the absence or presence of CXCL12. After 14-day culture, CD3−NK1.1+ NK cells were counted by flow cytometry; n = 4. *P < .05. (B) Sorted CD3−NK1.1+DX5− iNKs were cultured in medium containing IL-15 in the absence or presence of CXCL12. After 3-day culture, the number of CD3−NK1.1+DX5+ mNKs were measured by flow cytometry; n = 4. *P < .05.
In vitro effects of CXCL12 on generation of NK cells from LMPPs or iNKs. (A) Sorted Lin−Sca-1+c-kit+ (LSK) Flt3hi LMPPs were cultured in medium containing Flt3L, SCF, and IL-15 in the absence or presence of CXCL12. After 14-day culture, CD3−NK1.1+ NK cells were counted by flow cytometry; n = 4. *P < .05. (B) Sorted CD3−NK1.1+DX5− iNKs were cultured in medium containing IL-15 in the absence or presence of CXCL12. After 3-day culture, the number of CD3−NK1.1+DX5+ mNKs were measured by flow cytometry; n = 4. *P < .05.
NK cells are associated with CAR cells in bone marrow
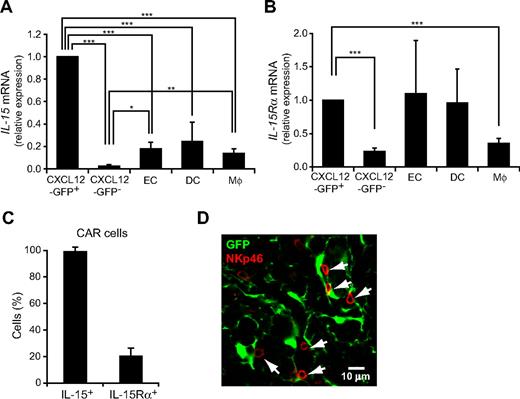
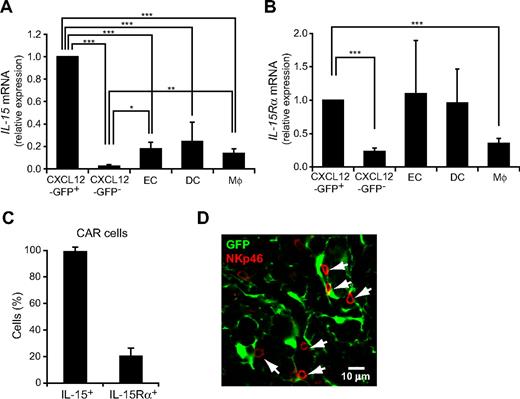
It has been shown previously that IL-15 is essential for the development and functional maturation of NK cells.12 Thus, the result that CXCL12 is predominantly expressed in a small population of reticular cells, termed CXCL12-abundant reticular (CAR) cells in the bone marrow,29,32 prompted us to examine the expression of IL-15 in CAR cells. It has been reported previously that IL-15 is expressed in bone marrow nonhematopoietic radiation-resistant cells, DCs, and activated monocytes.36,37 Quantitative RT-PCR analysis of bone marrow from mice with the GFP reporter gene knocked into the CXCL12 locus (CXCL12-GFP knock-in mice)28,29,32 revealed that the expression of IL-15 was considerably higher in CXCL12-GFPhi CAR cells than in CXCL12-GFP− nonhematopoietic cells, CD11chigh MHC class IIhigh DCs, and Gr-1loMac-1+ myeloid lineage cells (Figure 6A). In addition, it has been reported previously that the expressions of IL-15 and IL-15Rα by the same cells support NK-cell maintenance.38,39 Quantitative RT-PCR analysis of bone marrow from CXCL12-GFP knock-in mice revealed that IL-15Rα was expressed in CAR cells as well as CD11chigh MHC class IIhigh DCs, Gr-1loMac-1+ monocytes, and CD45−CD31+ endothelial cells (Figure 6B). Single-cell RT-PCR analysis revealed that all individual cells within the CAR cell population expressed IL-15 but only 26% of individual cells within the CAR cell population expressed IL-15Rα (Figure 6C). We next analyzed the association of NK cells with CAR cells in the bone marrow from pIpC-treated MxCre/CXCR4f/null-CXCL12-GFP knock-in mice and control CXCR4wt/wt-CXCL12-GFP knock-in mice. Immunohistochemical analysis with an antibody against NK-cell marker NKp4640 revealed that most NK cells were in contact with the processes of CAR cells but at a distance from c-kit+Sca-1+ primitive hematopoietic cells or pDCs in pIpC-treated MxCre/CXCR4f/null-CXCL12-GFP knock-in mice (data not shown) and control CXCR4wt/wt-CXCL12-GFP knock-in mice (280 of 341; 82%) (Figure 6D). These results suggest that CAR cells, which produce both CXCL12 and IL-15, act as a microenvironmental niche for NK-cell development, but CXCL12-CXCR4 signaling is not essential for the association of NK cells with CAR cells in the bone marrow.
The expression of IL-15 in CAR cells and their association with NK cells in bone marrow. (A-B) Quantitative RT-PCR analysis of mRNA expression of IL-15 (A) and IL-15Rα (B) in CXCL12-GFPhi (CAR) cells, CD45−CXCL12-GFP− nonhematopoietic cells, CD45−CD31+ endothelial cells (EC), CD11chigh MHC class IIhigh DCs, and Gr-1loMac-1+ myeloid lineage cells (Mϕ) in the bone marrow from wild-type mice or CXCL12-GFP knock-in mice. (C) Single-cell RT-PCR analysis of the frequencies of cells expressing IL-15 or IL-15Rα in sorted CAR cells; n = 3. (D) The bone marrow sections from CXCL12-GFP knock-in mice were stained with antibodies against NKp46 (red). Most NKp46+ NK cells are in contact with CAR cells (green). *P < .05. **P < .01. ***P < .001.
The expression of IL-15 in CAR cells and their association with NK cells in bone marrow. (A-B) Quantitative RT-PCR analysis of mRNA expression of IL-15 (A) and IL-15Rα (B) in CXCL12-GFPhi (CAR) cells, CD45−CXCL12-GFP− nonhematopoietic cells, CD45−CD31+ endothelial cells (EC), CD11chigh MHC class IIhigh DCs, and Gr-1loMac-1+ myeloid lineage cells (Mϕ) in the bone marrow from wild-type mice or CXCL12-GFP knock-in mice. (C) Single-cell RT-PCR analysis of the frequencies of cells expressing IL-15 or IL-15Rα in sorted CAR cells; n = 3. (D) The bone marrow sections from CXCL12-GFP knock-in mice were stained with antibodies against NKp46 (red). Most NKp46+ NK cells are in contact with CAR cells (green). *P < .05. **P < .01. ***P < .001.
Discussion
This study has demonstrated that CXCL12-CXCR4 chemokine signaling is essential for the development of NK cells in adults. Both T cells and NK cells are thought to be generated from HSCs and CLPs, which require CXCR4; however, the numbers of T-cell precursors were relatively unimpaired, but the numbers of NKPs and iNKs were reduced and the numbers of mNKs were more severely reduced in the bone marrow of CXCR4 conditionally deficient mice compared with control mice. Together with the findings that the numbers of mNKs were also reduced in the spleen and peripheral blood of CXCR4 conditionally deficient mice and that CXCL12 enhanced the generation of mNKs from iNKs in culture, our results suggest that CXCL12-CXCR4 signaling plays a critical role in the generation of NK lineage cells within the bone marrow. In addition, the results that the frequencies of iNKs incorporated BrdU over a 3-day period were decreased in the absence of CXCR4 suggest that CXCL12 promotes proliferation of iNKs in the marrow.
Furthermore, the possibility that the reduction in NK cells that is observed after CXCR4 deletion may be the result of a reduction in DC subsets that provide transpresented IL-15 for NK-cell development and homeostasis36-39 appears unlikely because the numbers of CXCR4-deficient NK cells were severely reduced in the bone marrow of chimeric mice reconstituted with both CXCR4-deficient and wild-type hematopoietic cells.
In contrast to the bone marrow, NK cells do not require CXCL12 in fetal ontogeny. This supports the idea that HSCs present in fetal life give rise to different sets of lymphohematopoietic progenitors that require different cytokines than HSCs present in adult bone marrow.
Although essential signals downstream of CXCR4 in hematopoiesis remain unclear, recent studies have suggested that phosphoinositide 3-kinases (PI3Ks), PI3Kδ and PI3Kγ, which catalyze the phosphorylation of the 3′ hydroxyl group of phosphoinositides, generating PI(3,4,5)P3 are activated by G protein βγ subunits and downstream effectors of CXCR4 in β-selection during T-cell development.41 Of note, mice lacking PI3K catalytic subunits, p110γ and p110δ, had normal numbers of B cells but severely reduced numbers of mNK in the bone marrow and spleen,42 suggesting that in NK-cell development, but not in B-cell development, PI3Kδ and PI3Kγ serve as critical downstream regulators of CXCR4 signaling in the bone marrow.
Although IL-15 is known to be essential for development and functional maturation of NK cells, cellular sources of IL-15 in the bone marrow during homeostasis remain unclear. On the other hand, it has been reported previously that IL-15 and its receptor IL-15Rα are coexpressed by the same cells, which transpresent IL-15 to NK cells during development,38,39 and that lethally irradiated IL-15Rα–deficient mice reconstituted with wild-type bone marrow cells had decreased numbers of NK cells, indicating that IL-15Rα in nonhematopoietic cells is essential for NK-cell development.43 In addition, although IL-15 is expressed in DCs and monocytes,36,37 the expression of IL-15Rα by DCs and monocytes was dispensable for NK-cell development in the bone marrow.44 Thus, the results that mRNA expression of IL-15 and CXCL12 was considerably higher in CAR cells than in other nonhematopoietic cells, that CAR cells express IL-15Rα and that most NK cells were in contact with the processes of CAR cells suggest that CAR cells provide IL-15 and CXCL12 and function as a niche for NK-cell development in the bone marrow. In contrast to IL-15 and CXCL12, IL-15Rα was expressed in only 26% of individual CAR cells, raising the possibility that CAR cells expressing IL-15Rα create a specific niche for NK cells. Together with previous studies, our findings suggest that CAR cells function as niches for various types of hematopoietic cells, including HSCs, B cells, pDCs, and NK cells in the bone marrow. However, histologic analysis revealed that NK cells were not located close to c-kit+Sca-1+ primitive hematopoietic cells or pDCs, raising the possibility that CAR cells are a common cellular component of distinct specific niches for HSCs, B cells, pDCs and NK cells.
This study demonstrates the novel role of CXCL12-CXCR4 chemokine signaling in immune cell development. Further studies will be needed to determine how the generation of NK cells, B cells, and pDCs is regulated in the presence of CXCL12 in niches in which CAR cells are implicated as a key component.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank H. Kohara (Institute for Frontier Medical Sciences, Kyoto University) for technical assistance.
This work was supported by the Ministry of Education, Science, Sports and Culture of Japan and the Uehara Memorial Foundation.
Authorship
Contribution: M.N. and T.N. designed and performed the experiments, analyzed the data, and prepared the paper; Y.O. and T.S. performed the experiments and analyzed the data; S.O. and N.F. contributed materials and tools; and T.N. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Nagasawa, Department of Immunobiology and Hematology, Institute for Frontier Medical Sciences, Kyoto University, 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: tnagasa@frontier.kyoto-u.ac.jp.