Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) is a causative agent of adult T-cell leukemia and HTLV-1–associated myelopathy/tropical spastic paraparesis. HTLV-1–associated myelopathy/tropical spastic paraparesis is a chronic inflammatory disease characterized by loss of motor movement in response to spinal marrow cell destruction by T lymphocytes. To perform their cellular function, T cells need to be activated by antigen-presenting cells, such as dendritic cells (DCs). The aim of this work was to analyze DC differentiation and activation from monocytes of HTLV-1–infected individuals. We demonstrated that monocytes from HTLV-1–infected patients who had been stimulated to differentiate had an impaired loss of CD14 expression, expressed low levels of CD1a, and maintained secretion of tumor necrosis factor-α compared with monocytes from noninfected donors. We further evaluated DC activation by tumor necrosis factor-α. We observed that in response to activation, DCs that were derived from noninfected donors had an increase in the percentage of CD83+, CD86+, and human leukocyte antigen-DR+ cells, whereas in DCs derived from HTLV-1–infected patients, the percentage of CD83+, CD86+, and human leukocyte antigen-DR+ cells remained similar to that of nonactivated cells. Moreover, these cells had an impaired capacity to stimulate allogeneic T lymphocytes. We demonstrated that DC maturation was altered in HTLV-1–infected patients, which could contribute to the development of HTLV-1–associated diseases.
Introduction
The human T-cell lymphotropic virus type 1 (HTLV-1) is a Deltaretrovirus member of the Orthoretrovirinae subfamily.1 It has been estimated that HTLV-1 infects 10-20 million people worldwide, and high-prevalence areas include the tropical regions (Central Africa, Latin America) and southern Japan.2 In Brazil, the prevalence of HTLV-1 infection among blood donors is approximately 0.45%,3 which represents approximately 2 million infected people. It is estimated that 95% of HTLV-1–infected individuals are asymptomatic carriers. HTLV-1 is recognized as the causative agent of the adult T-cell leukemia/lymphoma (ATLL) malignancy,4 and the chronic inflammatory diseases HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP),5 polymyositis, arthritis, and uveitis.6
HAM/TSP is a chronic, progressive, inflammatory, disabling disease characterized by demyelination and loss of axons, neuronal degeneration, and gliosis. The main area of neurodegeneration is the spinal cord. Clinically, the majority of the patients present with a slowly progressive spastic paraparesis with lower back pain and bowel, urinary, and sexual dysfunction.7,8
CD4+ T lymphocytes are the predominant targets of HTLV-1 infection, but it has been shown that CD8+ T cells are also infected.9,10 The HTLV-1 infection induces the activation of CD4+ T lymphocytes, which leads to spontaneous proliferation11 ; it also promotes the expression of various cytokines and their receptors12,13 and induces abnormal expression of genes involved with apoptosis, cell cycle, and transcription factors.14,15
The risk of HAM/TSP disease is positively correlated with the magnitude of the proviral load in the blood.16 The control of HTLV-1 infection depends on CD8+ T lymphocytes, which are responsible for infected cell elimination.17 The individual immune response induced by HTLV-1 infection is linked directly to the susceptibility or resistance to development of HTLV-1–associated diseases. An efficient cytotoxic response is capable of controlling the proviral load and prevents disease, but inefficient cytotoxic activity leads to an increase of the infected cell number and exacerbation of the inflammatory state, which induces lesions of the central nervous system.18 The reasons why the HTLV-1–specific immune response is not always effective in preventing disease in HTLV-1 patients need to be established.
Dendritic cells (DCs) are specialized in capturing antigens, displaying them to lymphocytes, and providing signals that stimulate the proliferation and differentiation of lymphocytes. DCs are heterogeneous. There are 2 main groups: conventional and plasmacytoid DCs.19 The former originate in the bone marrow and differentiate from a monocyte precursor.20 The capacity of DCs to activate and regulate T-cell responses is acquired during a complex differentiation and maturation program. We have hypothesized that alterations in these cells in HTLV-1 patients should be associated with a defective response. DCs obtained from ATLL patients had a phenotype that was closely associated with immune suppression, expressing abnormalities in CD1a, CD86, and human leukocyte antigen-DR (HLA-DR) molecules, as well as decreased phagocytic activity.21 On the other hand, DCs activated by the viral protein Tax express molecules associated with maturation and activation.22 Furthermore, Jain et al23 demonstrated that Tax increases the capacity of DCs to stimulate allogeneic T-cell proliferation. Considering the characteristics of HAM/TSP and the importance of clarifying the role of DCs in this context, the aim of the present study was to investigate DC maturation from monocytes obtained from HTLV-1–infected patients.
Methods
Subjects
The ethics committee of the Instituto de Pesquisa Clínica Evandro Chagas approved the study protocol, and informed consent was obtained in accordance with the Declaration of Helsinki from HTLV-1–infected individuals (Table 1) attending the HTLV-1 outpatient clinic at Instituto de Pesquisa Clínica Evandro Chagas, Rio de Janeiro, Brazil. This study involved 22 HAM/TSP patients, 3 asymptomatic patients, and 15 noninfected individuals. The diagnosis of HAM/TSP was made according to World Health Organization criteria. Noninfected control subjects were matched according to age and sex.
Monocyte isolation and DC differentiation
A 20-mL peripheral blood sample was obtained from volunteers with sodium heparin (Roche) used as an anticoagulant. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (GE Healthcare Life Sciences) density gradient centrifugation and then washed 3× with saline solution. The cells were maintained in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (Gibco Life Technologies), 50μM β-mercaptoethanol, 60 mg/mL penicillin, and 100 mg/mL streptomycin (Sigma-Aldrich), pH 7.4. For fresh monocytes (day 0), PBMCs were seeded at a concentration of 1 × 107 cells per well for 2 hours in 12-well plates. For DC differentiation, PBMCs were seeded at a concentration of 5 × 106 cells per well for 2 hours in 24-well plates at 37°C in a 5% CO2 atmosphere. Afterward, nonadherent cells were removed by extensive washing. Adherent cells were used in some experiments (fresh monocytes) or cultured in RPMI (Sigma-Aldrich) supplemented with 10% fetal bovine serum (500 μL final volume) with or without 50 ng/mL granulocyte/macrophage colony-stimulating factor (GM-CSF; PeproTech) and 50 ng/mL interleukin-4 (IL-4; PeproTech) for 5 days at 37°C in a 5% CO2 atmosphere.
In some experiments, 1 μg/mL monoclonal neutralizing anti–tumor necrosis factor-α (TNF-α; R&D Biosystems) was added to monocytes on the first day of culture and on the third day to neutralize TNF-α. TNF-α (20 ng/mL; R&D Biosystems) added on the first day of culture was used as a control for TNF-α activity and neutralization.
DC activation
Immature DCs obtained after 5 days of culture had their culture medium replaced with fresh medium that contained GM-CSF and IL-4 in the presence or absence of 50 ng/mL TNF-α (PeproTech) for an additional 48 hours under the same conditions.
Phenotypic characterization
To determine CD14, CD1a, CD16, CD56, CD83, CD86, and HLA-DR expression in monocytes after DC differentiation or activation, cells were collected and incubated for 10 minutes with 5% PBS plus fetal calf serum solution. Cells were stained for 30 minutes at 4°C with fluorescein isothiocyanate–conjugated anti-CD14, phycoerythrin (PE)–conjugated anti-CD1a, PE-conjugated anti-CD16, PE-conjugated anti-CD56, PE-conjugated anti–HLA-DR, PE-conjugated anti-CD86, and PE-conjugated anti-CD83 monoclonal antibodies (all from BD Biosciences). After the incubation period, cells were washed with PBS and analyzed by flow cytometry (FACScan; Becton Dickinson). Ten thousand cells were acquired on the basis of forward and side scatter. Data analyses were performed with WinMDI 2.9 software.
Cell sorting
Fresh monocytes and cultured cells were stained as described in “Phenotypic characterization” with fluorescein isothiocyanate–conjugated anti-CD14, PE-conjugated anti-CD1a, PE-conjugated Cy5.5 anti-CD8, and anti-CD4 APC (all from BD Biosciences) monoclonal antibodies. Sorting of fresh monocytes and cells cultured in the presence or absence of cytokines was performed with a MoFlo flow cytometer (DakoCytomation) and EPICS ALTRA (Beckman Coulter) with Summit software (DakoCytomation).
Detection of HTLV-1 infection and HTLV-1 proviral load quantification
A real-time polymerase chain reaction (SmartCycler; Cepheid) assay was performed with a Puregene DNA Isolation Kit (Gentra) and TaqMan system (Applied Biosystems). Standard curves were generated by amplification of a β-globin gene fragment and, for HTLV-1 proviral load, the pX region (Tax gene) fragment from a cell line that contained a single copy of HTLV-1 provirus (TARL-2).24 The primer set for the HTLV-1 pX region was 5′-CGGATACCCAGTCTACGTGT-3′ and 5′-GAGCCGATAACGCGTCCATCG-3′, and for β-globin, it was 5′-GCAAGAAAGTGCTCGGTGC-3′ and 5′-TCACTCAGTGTGGCAAAGGTG-3′. The TaqMan fluorescent probe for the HTLV-1 pX region was 5′-FAMACGCCCTACTGGCCACCTGTC-TAMRA-3′, and for β-globin, it was 5′-FAM–TAGTGATGGCCTGGCTCACCTGGAC–TAMRA-3′. Polymerase chain reaction was performed with 200 ng of DNA with 12.5 μL of TaqMan Universal PCR Master Mix (Applied Biosystems), 15 pmol for pX primers, 30 pmol for β-globin primers, and 5 pmol and 2.5 pmol of the fluorescent probes. The HTLV-1 proviral load was calculated as follows: number of copies per 100 cells = [Tax copies (β-globin copies/2)] × 100. The lower limit of detection was 1 copy per 104 cells.
Cytokine production
Supernatants obtained from DC differentiation and activation cultures were collected and stored at −20°C until use. Interleukin-6 (IL-6), TNF-α, transforming growth factor-β, and interleukin-10 (IL-10) concentrations were measured with enzyme-linked immunosorbent assay (Duo-Set kits; R&D Systems) according to the manufacturer's instructions. Optic density was read at 450 nm in a microplate reader (Sunrise Basic; Tecan).
Cell morphology
For cell morphology assessment, DCs were plated in glass coverslips at 5 × 106 cells per well. After the differentiation process, cell images were captured through phase-contrast microscopy with magnification objectives ×40. For cell morphology visualization, cells were stained previously with a PanOptic kit according to the manufacturer's instructions (Laborclin). The image analysis system consisted of a light microscope (Zeiss Axioplan 2; Zeiss) and a charge-coupled device color camera (XS; SIS) connected to a computer. All images were stored as TIFF files. Image analysis was performed with Image-Pro Plus 4.5 software (Media Cybernetics).
Mixed lymphocyte reaction
After 48 hours of activation, DCs were tested for allostimulatory ability. A total of 104 uninfected lymphocytes were cultured in 96-well microplates (round-bottomed) with different concentrations of allogeneic DCs (1:10; 1:100) at 37°C in a 5% CO2 atmosphere. Thymidine incorporation was measured on day 5 by 6-hour pulse with [3H] thymidine (1 Ci/well; Amersham Life Sciences) and then harvested with a multiwell cell harvester. [3H] thymidine incorporation was evaluated by liquid scintillation counting with a TriCarb 1600CA (Packard Inc) counter.
Statistical analysis
Statistical analysis was performed by Mann-Whitney U test. Values of P ≤ .05 were considered statistically significant with Prism 5 software (GraphPad).
Results
Monocyte morphology
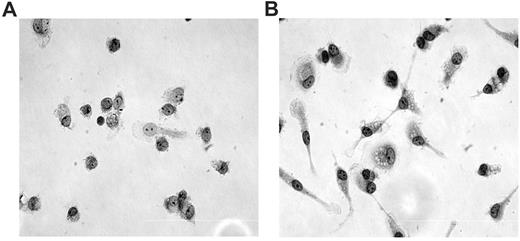
The first step was to analyze the morphology of monocytes obtained from noninfected and HTLV-1–infected donors. After 5 days in culture, we observed that monocytes obtained from noninfected donors had a classic cellular morphology of round cells with a large nucleus and sparse chromatin (Figure 1A). However, monocytes from HTLV-1–infected patients were adherent large cells with an elongated shape and spiny protrusions, and they demonstrated an increased intracellular complexity (Figure 1B). The expression of CD11b and CD86 in monocytes from noninfected individuals and HTLV-1–infected individuals was similar, despite the presence of morphologic features frequently related to cellular activation observed in the latter cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Monocyte morphology. Monocytes were cultured with medium and 10% fetal calf serum over slide glass for 5 days at 37°C with 5% CO2. After that, cells were stained with a PanOptic kit. (A) Monocytes obtained from noninfected donors; (B) monocytes obtained from HTLV-1–infected donors. Original magnification ×40 objective, visualized under inverted phase-contrast microscopy.
Monocyte morphology. Monocytes were cultured with medium and 10% fetal calf serum over slide glass for 5 days at 37°C with 5% CO2. After that, cells were stained with a PanOptic kit. (A) Monocytes obtained from noninfected donors; (B) monocytes obtained from HTLV-1–infected donors. Original magnification ×40 objective, visualized under inverted phase-contrast microscopy.
Monocytes from HTLV-1–infected individuals have a reduced capacity for DC differentiation in vitro
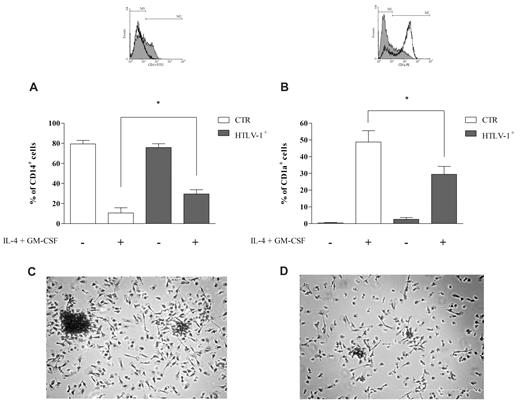
The morphology results led us to analyze the potential of monocytes obtained from HTLV-1–infected and noninfected individuals to convert into DCs. Peripheral blood monocytes can differentiate into DCs in vitro in the presence of IL-4 and GM-CSF. This process is characterized by down-regulation of CD14 expression and acquisition of CD1a expression in immature DCs.25 To investigate the capacity for differentiation into DCs in vitro, we evaluated the expression of CD14 and CD1a. Unstimulated monocytes obtained from noninfected and infected individuals expressed similar levels of CD14 and CD1a (Figure 2A-B). When monocytes from noninfected donors were stimulated to differentiate with IL-4 and GM-CSF, we observed a 90% decrease in the percentage of CD14+ cells (Figure 2A); in addition, 50% of these cells became CD1a+ (Figure 2B). However, when monocytes from HTLV-1–infected donors were cultivated with IL-4 and GM-CSF, the percentage of CD14+ cells decreased to a lesser degree than in control cells (approximately 70%), and only 30% of these cells became CD1a+ (Figure 2), which suggests that monocytes from infected HTLV-1 individuals have a reduced capacity for DC differentiation in vitro.
Monocytes from HTLV-1–infected individuals have a reduced capacity for DC differentiation in vitro. Cells were incubated with or without IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, CD14 and CD1a expression was analyzed by flow cytometry. Data in bars are expressed as the percentage of CD14+ cells (A) or CD1a+ cells (B). Open bars represent cells obtained from noninfected donors (CTR; n = 8), and shaded bars represent cells obtained from HTLV-1–infected donors (n = 12). Data are mean ± SD of 8 independent experiments. *Significantly different from control (P < .05). (Inset) Representative experiment showing CD14 and CD1a expression patterns in cells from noninfected donors (white) and HTLV-1–infected donors (gray; region M2 comprises positive cells). Cells were stained with a PanOptic kit. (C) Monocytes obtained from noninfected donors cultivated with IL-4 plus GM-CSF; (D) monocytes obtained from HTLV-1–infected donors cultivated with IL-4 plus GM-CSF. Original magnification ×20 objective, visualized under inverted phase-contrast microscopy.
Monocytes from HTLV-1–infected individuals have a reduced capacity for DC differentiation in vitro. Cells were incubated with or without IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, CD14 and CD1a expression was analyzed by flow cytometry. Data in bars are expressed as the percentage of CD14+ cells (A) or CD1a+ cells (B). Open bars represent cells obtained from noninfected donors (CTR; n = 8), and shaded bars represent cells obtained from HTLV-1–infected donors (n = 12). Data are mean ± SD of 8 independent experiments. *Significantly different from control (P < .05). (Inset) Representative experiment showing CD14 and CD1a expression patterns in cells from noninfected donors (white) and HTLV-1–infected donors (gray; region M2 comprises positive cells). Cells were stained with a PanOptic kit. (C) Monocytes obtained from noninfected donors cultivated with IL-4 plus GM-CSF; (D) monocytes obtained from HTLV-1–infected donors cultivated with IL-4 plus GM-CSF. Original magnification ×20 objective, visualized under inverted phase-contrast microscopy.
When monocytes are stimulated with IL-4 and GM-CSF, cells progressively acquire DC morphology. After 5 days in culture with both cytokines, adherent clusters were visible, and the cells displayed cytoplasmic processes or veils (Figure 2C). However, these features associated with DC appearance were not evident in monocytes from HTLV-1–infected donors stimulated with both cytokines (Figure 2D).
Cytokine secretion during DC differentiation
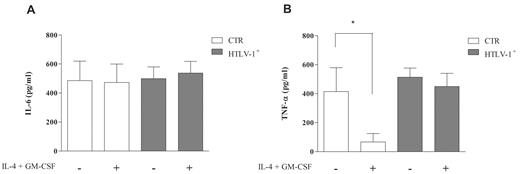
One of the several changes monocytes undergo during differentiation is related to the cytokine production pattern. After 5 days, supernatants were collected and assayed for IL-6 and TNF-α production. It was demonstrated that secretion of IL-6 was similar in monocytes and immature DCs from noninfected and HTLV-1–infected individuals (Figure 3A). However, we found TNF-α production in the supernatants of monocytes from noninfected individuals, whereas cells stimulated to differentiate produced lower levels of this cytokine (Figure 3B). In contrast, cells derived from HTLV-1–infected individuals that had been cultured with IL-4 plus GM-CSF displayed TNF-α levels similar to those of unstimulated monocytes (Figure 3B). These results suggested that the high levels of TNF-α produced by monocytes from HTLV-1–infected individuals after cytokine stimulation were in accordance with their phenotype (CD14+CD1alow) and their lower capacity for DC differentiation.
Cytokine secretion during DC differentiation. Cells were incubated with or without IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, supernatants were collected and assayed for IL-6 and TNF-α production by enzyme-linked immunosorbent assay. Data in bars are expressed as picomoles per milliliter of IL-6 (A) or TNF-α (B). Open bars represent supernatant obtained from culture of cells derived from noninfected individuals (CTR; n = 7), and shaded bars represent supernatant obtained from culture of cells derived from HTLV-1–infected donors (n = 15). Data are mean ± SD. *Significantly different from control (P < .05).
Cytokine secretion during DC differentiation. Cells were incubated with or without IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, supernatants were collected and assayed for IL-6 and TNF-α production by enzyme-linked immunosorbent assay. Data in bars are expressed as picomoles per milliliter of IL-6 (A) or TNF-α (B). Open bars represent supernatant obtained from culture of cells derived from noninfected individuals (CTR; n = 7), and shaded bars represent supernatant obtained from culture of cells derived from HTLV-1–infected donors (n = 15). Data are mean ± SD. *Significantly different from control (P < .05).
TNF-α neutralization during DC differentiation
When monocytes from noninfected individuals were stimulated to differentiate into DCs with IL-4 and GM-CSF, we observed a decrease in TNF-α secretion (Figure 3B). Because TNF-α reduction from DC supernatants as a result of diminished production or increased consumption is relevant to differentiation, we used a neutralizing antibody, anti–TNF-α, during the differentiation cultures.
Monocytes were incubated with IL-4 plus GM-CSF and anti–TNF-α, which was added to cultures twice on the first and third days. The presence of neutralizing antibody partially blocked the loss of CD14 expression and the expression of CD1a (supplemental Figure 2A), which indicates the importance of this cytokine for the differentiation process. This effect was proportional to the concentration of anti–TNF-α used (data not shown). To confirm the neutralization of TNF-α activity in the presence of anti–TNF-α, we evaluated CD83 expression induced by TNF-α. As shown in supplemental Figure 2B, the addition of neutralizing anti–TNF-α reversed the effect of TNF-α on CD83 induction.
Correlation between HTLV-1 proviral load and DC differentiation
The lower capacity for differentiation observed in cells obtained from HTLV-1–infected individuals raised the possibility that this impairment could be related to PBMC proviral load. Samples were analyzed with regard to the correlation between proviral load of PBMCs [from 0.75 to 25.98 Tax copies (β-globin copies/2) × 100] and the capacity for DC differentiation (percent of CD14+ and percent of CD1a+ cells). We did not find a significant linear correlation (r ≤ .2229 and r ≤ .0049; Figure 4A and 4B, respectively), which indicates that the higher proviral load did not translate to a lesser capacity for differentiation.
Correlation between HTLV-1 proviral load and DC differentiation. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, CD14 and CD1a expression was analyzed by flow cytometry. HTLV-1 proviral load was performed by real-time polymerase chain reaction and was calculated as follows: number of copies per 100 cells = [Tax copies (β-globin copies/2)] × 100. (A-B) Linear regression analysis was used to test the correlation between HTLV-1 proviral DNA load (measured in PBMCs; x-axis) and the percentage of CD14+ cells (A) or CD1a+ cells (B; y-axis). Cells were obtained from HTLV-1–infected patients and purified with a MoFlo sorter configured for high-speed sorting. (C-D) Representative gels of HTLV-1 Tax sequence in fresh monocytes and monocytes cultivated with or without IL-4 plus GM-CSF for 5 days. (C) First-round polymerase chain reaction with DNA extracted from 1 donor (patient 21084; lanes 2-5). Second-round polymerase chain reaction with DNA extracted from the same donor (lanes 8-11). L indicates ladder, 50 bp. Lanes 5 and 11 are CD4+ cells obtained from day 0, fresh sample; lanes 4 and 10, monocytes obtained from day 0, fresh sample; lanes 2 and 8, monocytes isolated after 5 days in culture; lanes 3 and 9, monocytes isolated after 5 days in culture with IL-4 and GM-CSF; and lane 6, negative control (H2O). (D) Polymerase chain reaction with DNA extracted from 1 donor (patient 23856; lanes 14-16). L indicates ladder, 50 bp. Lane 14 represents monocytes obtained from day 0, fresh sample; lane 15, monocytes isolated after 5 days in culture; and lane 16, monocytes isolated after 5 days in culture with IL-4 and GM-CSF. As a control, the β-globin gene was also investigated (below samples in panels C and D).
Correlation between HTLV-1 proviral load and DC differentiation. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, CD14 and CD1a expression was analyzed by flow cytometry. HTLV-1 proviral load was performed by real-time polymerase chain reaction and was calculated as follows: number of copies per 100 cells = [Tax copies (β-globin copies/2)] × 100. (A-B) Linear regression analysis was used to test the correlation between HTLV-1 proviral DNA load (measured in PBMCs; x-axis) and the percentage of CD14+ cells (A) or CD1a+ cells (B; y-axis). Cells were obtained from HTLV-1–infected patients and purified with a MoFlo sorter configured for high-speed sorting. (C-D) Representative gels of HTLV-1 Tax sequence in fresh monocytes and monocytes cultivated with or without IL-4 plus GM-CSF for 5 days. (C) First-round polymerase chain reaction with DNA extracted from 1 donor (patient 21084; lanes 2-5). Second-round polymerase chain reaction with DNA extracted from the same donor (lanes 8-11). L indicates ladder, 50 bp. Lanes 5 and 11 are CD4+ cells obtained from day 0, fresh sample; lanes 4 and 10, monocytes obtained from day 0, fresh sample; lanes 2 and 8, monocytes isolated after 5 days in culture; lanes 3 and 9, monocytes isolated after 5 days in culture with IL-4 and GM-CSF; and lane 6, negative control (H2O). (D) Polymerase chain reaction with DNA extracted from 1 donor (patient 23856; lanes 14-16). L indicates ladder, 50 bp. Lane 14 represents monocytes obtained from day 0, fresh sample; lane 15, monocytes isolated after 5 days in culture; and lane 16, monocytes isolated after 5 days in culture with IL-4 and GM-CSF. As a control, the β-globin gene was also investigated (below samples in panels C and D).
According to some authors, HTLV-1 may infect monocytes and DCs in vivo and in vitro.26-30 Thus, we explored whether the impaired differentiation of monocytes from HTLV-1–infected individuals was related to the infection of these precursors or even the infection of the cells during the culture period. Fresh monocytes were positive for HTLV-1 in 40% of samples from HTLV-1–seropositive individuals (PBMC proviral load 4.62 to 21.43). Of note, all of the samples of monocytes that were evaluated exhibited impaired differentiation to DCs, although only some of the samples had monocytes that were positive for HTLV-1. The infection was detected in fresh monocytes and was maintained in monocytes that were cultivated with or without IL-4 and GM-CSF for 5 days (Figure 4C), and it was not detected in fresh or cultivated monocytes (Figure 4D). These findings suggest that the differences observed (impaired differentiation) were influenced by conditions other than the infected state of monocytes.
Monocyte subpopulations
Randolph et al31 showed that the CD16+ subset of blood monocytes has the propensity among monocytes to develop into DCs. In this context, the disequilibrium in subsets could affect DC differentiation. We decided to verify whether monocytes from HTLV-1–infected individuals have alterations in the referred subset.
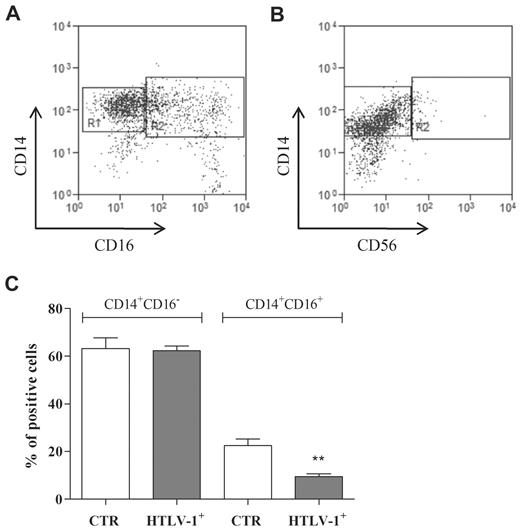
Figure 5 depicts a representative experiment of double staining for CD14 and CD16 (Figure 5A) that indicates the regions used for CD14+CD16− and CD14+CD16+ determination (R1 and R2, respectively) and double staining for CD14 and CD56 (Figure 5B) that shows that the population evaluated was negative for the natural killer cell marker CD56. It was verified from the data presented in Figure 5C that monocytes from HTLV-1–infected individuals had a decreased percentage of CD16+ cells compared with monocytes from noninfected individuals.
Monocyte subpopulations. Freshly isolated monocytes were obtained from noninfected donors and HTLV-1–infected donors. CD14 and CD16 expression was analyzed by flow cytometry. Representative dot plots showing (A) CD14 and CD16 (R1: CD14+CD16−, R2: CD14+CD16+) and (B) CD14 and CD56 (R1: CD14+CD56−, R2: CD14+CD56+) expression patterns. Data in bars (C) are expressed as percentage of positive cells. Data are mean ± SEM of 6 independent experiments. **Significantly different (P < .01) from control (CTR; CD14+CD16+).
Monocyte subpopulations. Freshly isolated monocytes were obtained from noninfected donors and HTLV-1–infected donors. CD14 and CD16 expression was analyzed by flow cytometry. Representative dot plots showing (A) CD14 and CD16 (R1: CD14+CD16−, R2: CD14+CD16+) and (B) CD14 and CD56 (R1: CD14+CD56−, R2: CD14+CD56+) expression patterns. Data in bars (C) are expressed as percentage of positive cells. Data are mean ± SEM of 6 independent experiments. **Significantly different (P < .01) from control (CTR; CD14+CD16+).
CD1a expression remained down-regulated during DC activation
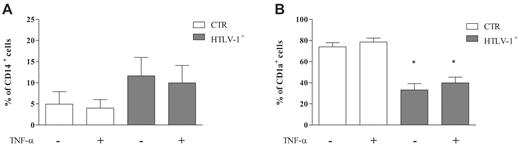
Because monocyte differentiation into immature DCs in HTLV-1–infected individuals was impaired, we decided to evaluate DC activation. Mature DCs can be generated from peripheral blood monocytes after culture with combinations of GM-CSF, IL-4, and TNF-α.32 DCs obtained after 5 days of culturing had their culture medium replaced by fresh medium that contained GM-CSF and IL-4 in the presence or absence of TNF-α. We observed that the addition of TNF-α did not alter CD14 or CD1a expression. After 7 days of culturing, cells derived from HTLV-1–infected donors had low levels of CD14 expression similar to HTLV-1–noninfected individuals (Figure 6A). However, the expression of CD1a remained down-regulated in cells from HTLV-1–infected donors (Figure 6B), which suggests that in the presence of GM-CSF and IL-4 for a further 48 hours, these cells maintained the altered phenotype observed during differentiation.
CD1a expression remained down-regulated during DC activation. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, cells were incubated in the presence or absence of TNF-α for 48 hours at 37°C in a 5% CO2 atmosphere. Next, CD14 and CD1a expression was analyzed by flow cytometry. Data in bars are expressed as percentage of CD14+ cells (A) or CD1a+ cells (B). Open bars represent cells obtained from noninfected donors (CTR; n = 8), and shaded bars represent cells obtained from HTLV-1–infected donors (n = 12). Data are mean ± SD of 8 independent experiments. *Significantly different from control (P < .05).
CD1a expression remained down-regulated during DC activation. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, cells were incubated in the presence or absence of TNF-α for 48 hours at 37°C in a 5% CO2 atmosphere. Next, CD14 and CD1a expression was analyzed by flow cytometry. Data in bars are expressed as percentage of CD14+ cells (A) or CD1a+ cells (B). Open bars represent cells obtained from noninfected donors (CTR; n = 8), and shaded bars represent cells obtained from HTLV-1–infected donors (n = 12). Data are mean ± SD of 8 independent experiments. *Significantly different from control (P < .05).
DCs derived from HTLV-1–infected individuals have a reduced capacity for activation in vitro
Mature DCs can be derived from blood monocytes under the influence of specific cytokines that induce the increased expression of many surface proteins, such as CD83, CD86, and HLA-DR.32 To investigate the activation of immature DCs differentiated from monocytes (noninfected and HTLV-1–infected donors), these cells were stimulated with TNF-α, and the expression of CD83, CD86, and HLA-DR was analyzed. Activated DCs derived from HTLV-1–noninfected donors expressed higher levels of CD83 than unstimulated cells (Figure 7A,D). However, when CD14− cells from HTLV-1–infected patients were used, the expression of CD83 was not altered in response to activation (Figure 7A,D).
DCs derived from HTLV-1–infected individuals have a reduced capacity for activation in vitro. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, cells were incubated in the presence or absence of TNF-α for 48 hours at 37°C in a 5% CO2 atmosphere. After 7 days, CD83, CD86, and HLA-DR expression was analyzed by flow cytometry. Data in bars are expressed as percentage of CD14−CD83+ cells (A), CD14−CD86+ cells (B), or CD14−HLA-DR+ cells (C). Open bars represent cells obtained from noninfected donors (CTR; n = 10), and shaded bars represent cells obtained from HTLV-1–infected donors (n = 12). Data are mean ± SD of 9 independent experiments. * and ** Indicate significantly different from DCs unstimulated with TNF-α (P < .05). (D) Representative experiment showing CD83, CD86, and HLA-DR expression patterns in cells from noninfected and HTLV-1–infected donors (region M2 comprises positive cells).
DCs derived from HTLV-1–infected individuals have a reduced capacity for activation in vitro. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, cells were incubated in the presence or absence of TNF-α for 48 hours at 37°C in a 5% CO2 atmosphere. After 7 days, CD83, CD86, and HLA-DR expression was analyzed by flow cytometry. Data in bars are expressed as percentage of CD14−CD83+ cells (A), CD14−CD86+ cells (B), or CD14−HLA-DR+ cells (C). Open bars represent cells obtained from noninfected donors (CTR; n = 10), and shaded bars represent cells obtained from HTLV-1–infected donors (n = 12). Data are mean ± SD of 9 independent experiments. * and ** Indicate significantly different from DCs unstimulated with TNF-α (P < .05). (D) Representative experiment showing CD83, CD86, and HLA-DR expression patterns in cells from noninfected and HTLV-1–infected donors (region M2 comprises positive cells).
In addition, we analyzed the expression of the costimulatory molecules CD86 and HLA-DR. DCs from noninfected donors activated with TNF-α displayed increased levels of the costimulatory molecules CD86 (approximately 65%) and HLA-DR (approximately 60%; Figure 7B-D) compared with immature DCs. Conversely, after TNF-α stimulation, CD14− cells derived from HTLV-1–infected patients had similar levels of CD86 and HLA-DR expression (Figure 7B-D) as unstimulated cells. Moreover, cells from HTLV-1–infected donors cultivated in the absence of TNF-α expressed higher levels of CD86 and HLA-DR than immature DCs derived from noninfected individuals. These results suggest that DCs derived from HTLV-1–infected individuals have a reduced capacity for activation in vitro.
Cytokine secretion during DC activation
The DC activation process includes the production of cytokines involved in the maturation of T cells and in the determination of their fate. After DC activation with TNF-α, supernatants were collected and assayed for IL-10 and transforming growth factor-β production (supplemental Figure 3). We observed that transforming growth factor-β secretion was similar in immature or activated DCs from noninfected and infected individuals (supplemental Figure 3A). IL-10 production was not modified when DCs were stimulated; however, secretion of IL-10 was slightly greater in DCs derived from monocytes of HTLV-1–infected individuals than in DCs from healthy individuals (supplemental Figure 3B).
DCs derived from HTLV-1–infected individuals have an impairment of allostimulation
Activated DCs are potent stimulators of T-cell proliferation. The function of activated DCs from HTLV-1–infected patients and noninfected individuals was compared by testing the stimulatory capacity of uninfected allogeneic T lymphocytes in primary mixed lymphocyte reactions. Activated DCs derived from healthy donors had an important stimulatory capacity (Table 2). However, when we compared this with the allostimulatory capacity of activated DCs derived from HTLV-1–infected individuals, a reduced stimulatory activity was observed (Table 2). These results suggest that the alterations in phenotype associated with DC maturation in cells from HTLV-1–infected patients reflect an impaired ability to induce allogeneic T-cell proliferation.
Discussion
The pathogenesis of inflammatory HTLV-1–associated diseases directly involves T-lymphocyte activation; however, the role of DCs in the modulation of this process is not entirely clear. We developed an important study in this area, because we analyzed cells obtained directly from HTLV-1–infected individuals. The present study demonstrates that monocytes obtained from HTLV-1–infected patients exhibit a reduced capacity for DC differentiation and activation. Monocytes from noninfected donors were stimulated to differentiate with IL-4 and GM-CSF, and as expected, they acquired an immature DC phenotype. However, monocytes from HTLV-1–infected individuals had a reduced capacity for DC differentiation in vitro, sustaining their CD14 expression and modestly increasing the expression of CD1a molecules. These results confirm observations reported by Makino et al,26 who showed that immature DCs obtained from 2 HAM/TSP patients expressed lower levels of CD1a+ than healthy donors. Furthermore, similar results were described by Al-Dahoodi et al,33 who demonstrated reduced CD1a expression in HTLV-1 carriers and ATLL patients. In the present study, important differences were observed in the morphology of monocytes from HTLV-1–infected individuals that suggested that cells from HTLV-1–infected patients may have alterations in their differentiation potential. A high HTLV-1 proviral load in mononuclear cells appears to be a risk factor for the development of HAM/TSP.16 HTLV-1 preferentially infects CD4+ T cells; however, some authors have demonstrated that HTLV-1 may infect monocytes and DCs in vivo.26-30 In accordance with this information, in the present work, HTLV-1–positive monocytes were detected in 40% of samples from HTLV-1–seropositive individuals. Because all the samples of monocytes that were evaluated for proviral load exhibited impairment of differentiation to DCs, although only some of the samples were HTLV-1–positive, it is likely that the differences observed were influenced by conditions other than the infected state of monocytes. In addition to infection in vivo, infection of HTLV-1 in vitro has been described in myeloid DCs, plasmacytoid DCs, and DCs differentiated from monocytes.26,29 It was of interest, therefore, to verify whether there was infection in vitro of cells in culture that correlated with differentiation impairment. However, infection was not detected in all cultures that showed impairments in differentiation. Moreover, we did not find a significant correlation between a high proviral load of PBMCs and the capacity for DC differentiation. These results corroborate data from Azakami et al,30 who observed that a low frequency of myeloid and plasmacytoid DCs was not correlated with proviral load, which suggests that proviral load and monocyte infection are not related to impairment of DC differentiation.
Some conditions have been discussed as important in DC differentiation. A subpopulation of monocytes, CD16+, has been revealed to be committed to DC differentiation, or at least to be more prone to DC differentiation.31 Thus, modifications in this subpopulation could translate to impairment of differentiation into DCs. In this regard, we showed that monocytes from HTLV-1–infected individuals had a decreased percentage of CD16+ cells compared with monocytes from noninfected individuals. This reinforces the idea that alterations in monocyte subpopulations interfere with DC differentiation.
It has been shown that monocytes derived from a special environment that consists of leukocytes that secrete cytokines could exhibit a lineage commitment.12,34-36 Chomarat et al37 demonstrated that IL-6 controls monocyte differentiation into macrophages at the expense of DCs. Indeed, elevated levels of IL-6 in the serum and cerebrospinal fluid of HAM/TSP patients have been observed.38 Moreover, Brito Melo et al39 demonstrated an elevated frequency of TNF-α–positive monocytes in HTLV-1–infected patients compared with noninfected individuals. In the present study, we observed that TNF-α secretion by monocytes from noninfected donors was abolished after IL-4 plus GM-CSF; however, TNF-α secretion by monocytes from HTLV-1–infected patients was maintained during DC differentiation. We then incubated monocytes from HTLV-1–infected patients with neutralizing anti–TNF-α to explore whether it would improve their differentiation. In contrast to what was expected, the presence of the neutralizing antibody impaired differentiation in cells from both noninfected and HTLV-1–infected patients. This result suggests that the decreased levels of TNF-α observed after DC differentiation from noninfected donors might be a result of increased consumption, and this feature appears to be important for the process; however, it was not possible to confirm this idea, because we did not study the kinetics of TNF-α production during differentiation.
Impairment of DC differentiation might lead to decreases in their frequency. In fact, previous studies reported that the number of peripheral myeloid and plasmacytoid DCs was significantly reduced in ATL,40 HIV-1 infection,41 and chronic inflammatory diseases such as Sjögren syndrome.42 Moreover, Azakami et al30 showed recently that the frequency of DCs in the peripheral blood of HAM/TSP and ATL patients is decreased.
There is scarce information about DCs from HTLV-1–infected individuals in relation to their response to activation stimuli. Normally, activated DCs express high cell-surface levels of major histocompatibility complex molecules, CD40, CD80, CD83, and CD86.32 Recently, Jain et al23 showed that Tax purified protein induced maturation of DCs generated from monocytes derived from noninfected individuals, but the expression of CD83 was lower than that induced by lipopolysaccharide. In the present study, TNF-α–activated DCs derived from noninfected donors expressed high levels of CD83; however, even in CD14− cells (immature DC phenotype) from HTLV-1–infected patients, the expression of CD83 (restricted to DC activation) did not change during TNF-α activation, which demonstrates that these cells have a reduced capacity for activation in vitro.
As expected, activated DCs from noninfected donors exhibited an up-regulation of CD86 and HLA-DR expression, whereas immature DCs from infected patients did not have any modulation of the expression of these molecules. This might be because the expression of CD86 and HLA-DR in immature DCs from HTLV-1–infected patients was higher than that of immature DCs from noninfected donors. An elevated expression of CD86 was previously observed in immature DCs from HAM/TSP patients, which corroborates the present results.26
Mature DCs have the ability to stimulate T cells. Previous reports demonstrated that DCs were involved in the spontaneous lymphocyte proliferation observed in HTLV-1–infected individuals.27,43 It has been demonstrated that DCs generated from monocytes from noninfected individuals that were stimulated with Tax purified protein induced an allogeneic mixed lymphocyte reaction to a greater degree than unstimulated cells.23,44 However, the present results demonstrated that monocytes derived from noninfected donors cultured with GM-CSF plus IL-4 plus TNF-α were more efficient in inducing the proliferation of noninfected allogeneic T cells than monocytes from HTLV-1–infected individuals. Similar results of allogeneic and autologous mixed lymphocyte reactions were observed in mature DCs derived from ATLL patients.21,33 In addition, in HIV infection, it was demonstrated that myeloid and plasmacytoid DCs show a diminished ability to stimulate allogeneic mixed lymphocyte reactions.45
Modifications that occur during DC development from precursors and lead to a different phenotype and function have a direct effect on T-lymphocyte responses. There is some evidence that suggests that the cytotoxic response is responsible for the control of proviral load and consequently the risk of HAM/TSP development.46,47 To control HTLV-1 infection, the efficacy of the cytotoxic T lymphocyte response depends on the percentage of T regulatory cells and their function. Recently, Toulza et al48 demonstrated that the percentage of CD4+FoxP3+ regulatory T cells in HTLV-1–infected individuals was higher than in noninfected individuals. It has been demonstrated that DCs are able to induce tolerance through T regulatory cell conversion from naive T cells or expansion of T regulatory cells. Initially, the tolerogenic ability of DCs was described in immature cells; however, some researchers have suggested that DC tolerance induction requires partial maturation.49,50 It is likely that the partial maturation of DCs observed in the present study in HTLV-1–infected individuals might be involved in generating the high percentage of CD4+FoxP3+ cells, with a significant impact on HAM/TSP pathogenesis.
In conclusion, the present study showed impaired DC differentiation and activation in HTLV-1–infected patients. This phenomenon could be an effect of the altered environment in which monocytes were originated, inducing a lineage commitment of these cells. DC development in such conditions can lead to tolerance and immune response evasion. Finally, the present study emphasizes the relevance of studying the relation between DC maturation and T-lymphocyte activation during the control of HTLV-1 infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Vivian M. Rumjanek from the Instituto de Bioquímica Médica of the Universidade Federal do Rio de Janeiro for helpful discussions and for providing some reagents for the study. We acknowledge Ramza C. Harab and Luciano de Oliveira from the Instituto de Pesquisa Clínica Evandro Chagas for technical support.
This work was supported by grants from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, the Academy of Sciences for the Developing World, the Fundação Oswaldo Cruz, and the Brazilian Ministry of Health. C.R.N. was the recipient of a post-PhD Research Fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Authorship
Contribution: C.R.N. analyzed the data and wrote the manuscript, and together with J.E.-L. designed and performed experiments; M.A.L. performed clinical work, supplied blood samples, and contributed to the writing of the manuscript; M.J.A.S. obtained ethics approval, designed some experiments, and recruited patients for the study; O.E. performed assays of HTLV-1 DC and monocyte proviral load; and A.C.C.L. supplied blood samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Professor Juliana Echevarria-Lima, Departamento de Imunologia, Instituto de Microbiologia Paulo de Góes, Centro de Ciências da Saúde, Bloco I, Sala I2-051, Universidade Federal do Rio de Janeiro, 21941-590, Rio de Janeiro, Brazil; e-mail: juechevarria@micro.ufrj.br.

![Figure 4. Correlation between HTLV-1 proviral load and DC differentiation. Cells were incubated with IL-4 plus GM-CSF (50 ng/mL) for 5 days at 37°C in a 5% CO2 atmosphere. After this period, CD14 and CD1a expression was analyzed by flow cytometry. HTLV-1 proviral load was performed by real-time polymerase chain reaction and was calculated as follows: number of copies per 100 cells = [Tax copies (β-globin copies/2)] × 100. (A-B) Linear regression analysis was used to test the correlation between HTLV-1 proviral DNA load (measured in PBMCs; x-axis) and the percentage of CD14+ cells (A) or CD1a+ cells (B; y-axis). Cells were obtained from HTLV-1–infected patients and purified with a MoFlo sorter configured for high-speed sorting. (C-D) Representative gels of HTLV-1 Tax sequence in fresh monocytes and monocytes cultivated with or without IL-4 plus GM-CSF for 5 days. (C) First-round polymerase chain reaction with DNA extracted from 1 donor (patient 21084; lanes 2-5). Second-round polymerase chain reaction with DNA extracted from the same donor (lanes 8-11). L indicates ladder, 50 bp. Lanes 5 and 11 are CD4+ cells obtained from day 0, fresh sample; lanes 4 and 10, monocytes obtained from day 0, fresh sample; lanes 2 and 8, monocytes isolated after 5 days in culture; lanes 3 and 9, monocytes isolated after 5 days in culture with IL-4 and GM-CSF; and lane 6, negative control (H2O). (D) Polymerase chain reaction with DNA extracted from 1 donor (patient 23856; lanes 14-16). L indicates ladder, 50 bp. Lane 14 represents monocytes obtained from day 0, fresh sample; lane 15, monocytes isolated after 5 days in culture; and lane 16, monocytes isolated after 5 days in culture with IL-4 and GM-CSF. As a control, the β-globin gene was also investigated (below samples in panels C and D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2010-03-272690/4/m_zh89991063790004.jpeg?Expires=1767753116&Signature=h7uzpVOM2xIbJJqTUrP2Binj0a8DYVtH2QzgMWy8kUo2gPad9fGS4VaYuqDRVFhK-MRwn42HdXnIWFs~kfV80udGbNJzEJVKWWMYt~g~O-qxLI1VdlBmwGR37akUOVPAZVCPDA2NJd~0qbKzrDeC-pbpChjTkH48lGU130SV18uJnOahztzj3gqCixrM5btDyWUU~9CRGR4TZSYbtSIPUkinBwZH~tD0q4EwaVGWc31WEpyQEdQvA0VRZWjLKIwee8~9wFDO3mC-N9L0ufytAiuPV4mf292Wtb4Rv~wMffCIEGOf5xB9XrF~Z3ba~ncmcvwYomXcaCAoriM4QTkNgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)