The study by Barsam et al of patients with immune thrombocytopenia (ITP) in this issue of Blood suggests that stimulation of megakaryopoiesis with thrombopoietin-receptor agonists does not always result in improvement of the platelet count because of ineffective thrombopoiesis.1
In the first issue of Blood published in 1946, Dameshek and Miller reported key findings for our current interpretation of the mechanisms of thrombocytopenia in ITP.2 They observed that the numbers of megakaryocytes in ITP were normal or increased, but only one-third or fewer of megakaryocytes showed evidence of platelet production. In addition, they also found that the numbers of a larger intermediate megakaryocyte, the promegakaryocyte, were decreased. They concluded that thrombocytopenia in ITP may result from a severe reduction in platelet production by megakaryocytes. Interestingly, effective platelet production appeared to increase after splenectomy.
The debate about the mechanisms of the thrombocytopenia in ITP, peripheral destruction of platelets versus impaired platelet production, was apparently settled in 1951 by Harrington's seminal experiment,3 unequivocally demonstrating that ITP is characterized by reduced platelet survival because of a humoral factor later identified as antibodies against platelet glycoproteins.
Platelet kinetic studies performed decades later using indium-111 (111In)–labeled autologous platelets revealed a more heterogenous scenario.4 While the platelet lifespan was markedly decreased in virtually all patients (with the exception of a few splenectomized patients), platelet turnover (a measure of platelet production) was frequently subnormal. It was therefore proposed that thrombocytopenia may result from both platelet destruction and antibody-mediated damage to megakaryocytes. Evidence to support the latter hypothesis has accumulated over time. Two studies in particular, by Chang et al5 and McMillan et al,6 support the view that autoantibodies in ITP suppress megakaryocyte production and maturation and platelet release. Electron microscopy studies have clarified some aspects of the autoantibody-induced damage in bone marrow megakaryocytes from patients with ITP. Extensive megakaryocytic abnormalities, often presenting with the features of nonclassic apoptosis, are consistently present in a significant percentage of all stages of ITP megakaryocyte.7 Furthermore, para-apoptotic changes could be induced in megakaryocytes derived from CD34+ cells grown in ITP plasma, suggesting that autoantibodies may initiate the cascade of programmed cell death.7 The role of T cells in this process is still uncertain, although there is evidence that ITP is associated with accumulation and activation of T cells in the bone marrow that occurs through increased VLA-4 and CX3CR1 expression.8
Because of the autoimmune nature of the disease, a wide range of immunosuppressive and immunomodulatory drugs have been used in patients with ITP. The mechanism of action of these therapeutics was generally inferred from knowledge of their activity in other systems, varying from inhibition of macrophage Fcγ receptor (FcγR) function to inhibition of antibody production by B-cell depletion. However, no systematic investigation of their effects on platelet production and destruction has been performed.
A conceptually different approach to the treatment of ITP has emerged in recent years with the introduction of second-generation thrombopoietin receptor (TPO-R) agonists, which aim at correcting the thrombocytopenia by stimulating platelet production through megakaryocyte proliferation and maturation. Two such agents, romiplostim and eltrombopag, have recently been licensed for use in this condition.
Barsam and colleagues report their investigation of the patterns of both platelet production and platelet destruction in patients with ITP undergoing various pharmacologic treatments, including eltrombopag.1 To this end, they determined the absolute immature platelet fraction A-IPF and plasma glycocalicin levels normalized to the individual platelet count (GC index; GCI) as measures of platelet production and platelet turnover, respectively. None of the patients responding to anti-FcγRIII antibody and IV anti-D, 2 of 8 responding to IVIG and one with IVIG/IVanti-D combined exhibited significant increases in the A-IPF. These results consolidate previous studies suggesting that inhibition of platelet destruction is the primary mechanism of action of these agents. There was, however, an inverse correlation between the A-IPF and the glycocalicin index, indicating a relationship between platelet production and destruction.
With regard to eltrombopag, while responders showed the expected increase of the A-IPF, there was no significant change of this parameter in nonresponders. Bone marrow histology in nonresponders showed features similar to responders, that is, increased megakaryocytes and megakaryocyte clustering, with only 1 patient exhibiting moderate fibrosis. Although platelet turnover was not assessed in these patients and although intramedullary platelet destruction may play a relevant role in causing thrombocytopenia, these findings suggest that the megakaryocytes were inhibited in either producing or releasing platelets.
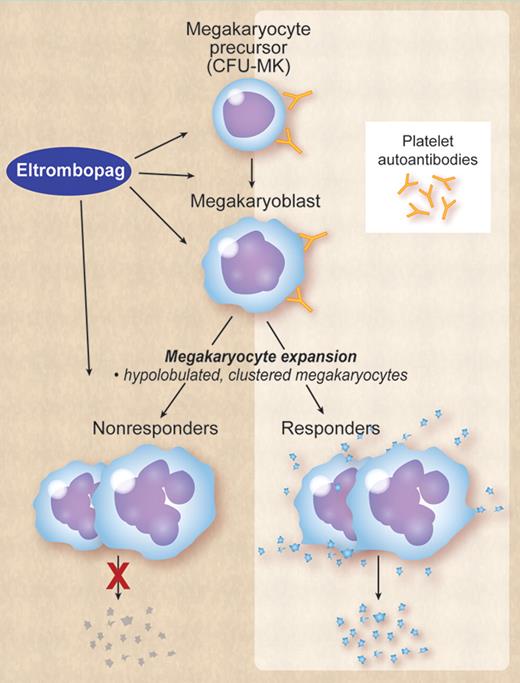
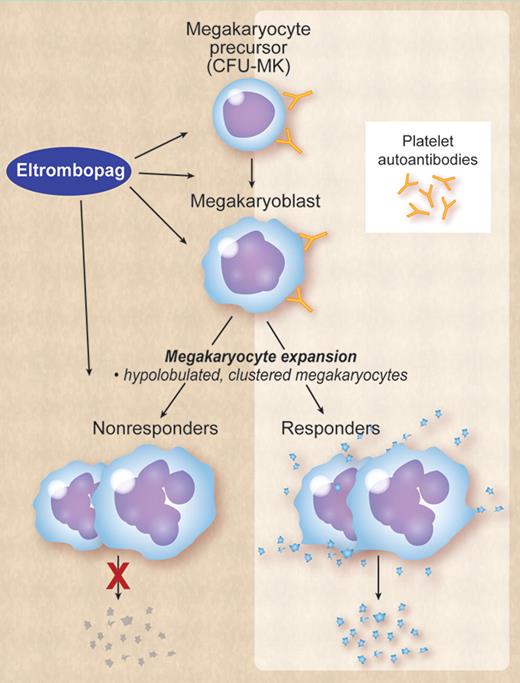
That said, by what specific mechanisms does eltrombopag stimulate thrombopoiesis in some patients with ITP but not in others? One plausible explanation is that in some patients proplatelet formation and release by megakaryocytes is completely inhibited by platelet antibodies and in some is not or only partially inhibited. Eltrombopag can still stimulate megakaryocyte proliferation in ITP, but like thrombopoietin has no effect on the final steps of thrombopoiesis, that is, proplatelet formation and release. Accordingly, conventional morphologic examination of the bone marrow will show similar features in both responders and nonresponders, the difference between the 2 being only functional: responders produce platelets, nonresponders do not (see figure). The extent of megakaryocyte inhibition may possibly depend on factors such as antibody specificity, affinity, and titer. For example, in vitro studies suggest that monoclonal antiplatelet antibodies have differing effects on in vitro proplatelet formation, with anti-GPIbα strongly inhibiting proplatelet formation; anti-GPIIb inducing a partial inhibition; and anti-GPIIIa having no effects.9
ITP is characterized by autoantibodies to platelet glycoproteins that also bind megakaryocytes and megakaryocyte precursors in the bone marrow, thereby impairing megakaryocyte maturation and proplatelet release. In some patients inhibition of proplatelet release is extreme, in others it is only partial or minimal. Eltrombopag or other TPO-R agonists can stimulate megakaryocyte proliferation and maturation, but has no effect on proplatelet formation and release. Both in responders and nonresponders the bone marrow will show an increased number of megakaryocytes, which tend to be clustered and have a hypolobulated nucleus. The difference between responders and nonresponders is only functional: responders produce platelets, nonresponders do not. Professional illustration by Debra Dartez.
ITP is characterized by autoantibodies to platelet glycoproteins that also bind megakaryocytes and megakaryocyte precursors in the bone marrow, thereby impairing megakaryocyte maturation and proplatelet release. In some patients inhibition of proplatelet release is extreme, in others it is only partial or minimal. Eltrombopag or other TPO-R agonists can stimulate megakaryocyte proliferation and maturation, but has no effect on proplatelet formation and release. Both in responders and nonresponders the bone marrow will show an increased number of megakaryocytes, which tend to be clustered and have a hypolobulated nucleus. The difference between responders and nonresponders is only functional: responders produce platelets, nonresponders do not. Professional illustration by Debra Dartez.
From a clinical perspective strategies to overcome resistance to TPO-R agonists may involve adding conventional immunosuppressive agents to inhibit autoantibody production (eg, azathioprine, as in the anecdotal case reported in the Barsam study). This may be a practical tip for doctors who in their clinics see patients responding poorly to the second generation thrombopoietic agents, as well as an idea for designing the next clinical trial with these agents in ITP.
Conflict-of-interest disclosure: The author has served as a consultant for Amgen, GlaxoSmithKline, and Suppremol and has participated on advisory boards and/or as a speaker at medical education events supported by Amgen, GlaxoSmithKline, Nycomed, Novo, Bayer, and Baxter. ■