Abstract
The requirement of c-Myb during erythropoiesis spurred an interest in identifying c-Myb target genes that are important for erythroid development. Here, we determined that the neuropeptide neuromedin U (NmU) is a c-Myb target gene. Silencing NmU, c-myb, or NmU's cognate receptor NMUR1 expression in human CD34+ cells impaired burst-forming unit-erythroid (BFU-E) and colony-forming unit-erythroid (CFU-E) formation compared with control. Exogenous addition of NmU peptide to NmU or c-myb siRNA-treated CD34+ cells rescued BFU-E and yielded a greater number of CFU-E than observed with control. No rescue of BFU-E and CFU-E growth was observed when NmU peptide was exogenously added to NMUR1 siRNA-treated cells compared with NMUR1 siRNA-treated cells cultured without NmU peptide. In K562 and CD34+ cells, NmU activated protein kinase C-βII, a factor associated with hematopoietic differentiation-proliferation. CD34+ cells cultured under erythroid-inducing conditions, with NmU peptide and erythropoietin added at day 6, revealed an increase in endogenous NmU and c-myb gene expression at day 8 and a 16% expansion of early erythroblasts at day 10 compared to cultures without NmU peptide. Combined, these data strongly support that the c-Myb target gene NmU functions as a novel cofactor for erythropoiesis and expands early erythroblasts.
Introduction
Erythropoiesis is the process by which hematopoietic cells mature into red blood cells. The early stages of erythropoiesis have been studied through colony-forming assays in which the cells first committed to erythropoiesis are burst-forming unit-erythroid (BFU-E) followed by colony-forming unit-erythroid (CFU-E).1 The CFU-Es undergo a series of cell divisions to give rise to mature erythroblasts that become hemoglobinized. Reticulocytes form after the nuclei extrude from the hemoglobinized erythroblasts. Finally, the reticulocytes give rise to red blood cells. Extrinsic signals mediated by cytokines and other factors in the microenvironment have been reported to be critical during red blood cell development. For instance, the interaction of stem cell factor (SCF) with c-Kit receptor,2 which is expressed in immature hematopoietic cells until the CFU-E stage,3 and the interaction of erythropoietin (EPO) with the EPO receptor (EPOR),4 which is expressed on erythroid progenitors until later erythroblast stages,5 are critical for erythroid progenitor cell survival, expansion, and differentiation.
Mouse models, in which transcription factor genes have targeted mutations, revealed that regulation of erythroid development is important at the transcription level.6-12 For example, the c-myb proto-oncogene encodes the c-Myb transcription factor that is expressed predominantly in immature hematopoietic cells, and as these cells mature, c-Myb expression declines. c-Myb has been reported to play an important role in cell survival, proliferation, and differentiation.13 Mice that are homozygous for the c-myb null allele die of severe anemia at embryonic day 15 (E15) because of diminished erythropoiesis in the fetal liver.12 In K562 cells engineered to express a tamoxifen-inducible dominant negative Myb (MERT), we identified the neuropeptide neuromedin U (NmU) as a novel candidate Myb target gene but did not demonstrate that Myb directly regulates NmU expression.14 An examination of NmU mRNA expression in nondiseased tissue revealed that it is expressed the highest in brain, gut, and bone marrow.15 Studies of NmU function in rat brain tissue have revealed that it decreases appetite and body weight16-20 and increases gross locomotor activity, body temperature, heat production, oxygen consumption, and bone mass.16,18,21 In the gut, NmU has been reported to regulate local blood flow and ion transport.22-24 Although NmU has been reported to stimulate cytokine production in the murine Th2-type T-cell clone D10.G4.125 and promote mast cell-mediated inflammation,26 the physiologic function of NmU during hematopoiesis in the bone marrow remains ill defined. Given that c-Myb is required during erythropoiesis and that NmU is a candidate Myb target gene, we postulated that c-Myb induces NmU expression in normal hematopoietic cells to facilitate erythropoiesis.
In this study, we demonstrate that NmU is a novel extrinsic factor that is important for the production of BFU-E and CFU-E from primary human CD34+ cells. We then determined that NmU activates protein kinase C-βII in CD34+ cells and that c-Myb not its homolog B-Myb directly regulates NmU gene expression. Finally, we provide evidence that NmU in combination with EPO expand early erythroblasts. Taken together, these data identify NmU as a c-Myb target gene in hematopoietic cells, which, when expressed, functions as a novel cofactor during the early stages of erythropoiesis.
Methods
Cells and culture conditions
K562 and K562-MERT cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone Laboratories). Primary human hematopoietic cells (CD34+) cells from normal donor cord blood were obtained from the University of Pennsylvania Stem Cell and Xenograft Core Facility. The cord blood samples were collected in accordance with the guidelines approved by the Institutional Review Board of the University of Pennsylvania. Human erythroid progenitors were derived from primary human CD34+ cells cultured in erythroid-inducing conditions as described27 except that EPO (2 U/mL; Amgen) without and with NmU (17μM; Invitrogen) were added at day 6 of culture. The cultured primary cells were harvested on days 0, 1, 2, 3, 6, 8, and 10 and washed in Dulbecco phosphate-buffered saline before the cell pellet was either processed to detect transferrin receptor (CD71) and glycophorin A (GlyA) expression by flow cytometry or stored at −80°C until further use. For luciferase assays, CD34+ cells were cultured for 8 days in IL-3 (10 ng/mL), IL-6 (10 ng/mL), and SCF (25 ng/mL) before nucleofecting the cells with the luciferase reporter construct.
CFU assays
Non-nucleofected and nucleofected CD34+ cells (1.5 × 103) were resuspended in 900 μL of IMDM supplemented with 20% of the serum substitute BIT9500 (StemCell Technologies) and the appropriate cytokines to stimulate BFU-E growth (EPO 2 U/mL, Amgen; and SCF 0.5 ng/mL, R&D Systems), CFU-E growth (EPO 2 U/mL, Amgen), or colony-forming unit–granulocyte, macrophage (CFU-GM) growth (IL-3 2 ng/mL and granulocyte-macrophage colony-stimulating factor [GM-CSF] 1 ng/mL; Amgen) without and with NmU (17μM; Invitrogen) or with neurotensin (17μM; Sigma-Aldrich). The NmU used in these experiments and the immunofluorescent microscopy assays was custom synthesized with the amino acid sequence: FRVDEEFQSPFASQSRGYFLFRPRN-NH2 (Invitrogen). BFU-E, CFU-E, and CFU-GM were enumerated on days 14, 7, and 11, respectively.
Silencing endogenous expression of c-myb, B-myb, NmU, and NMUR1
Short interfering RNA (siRNA) duplexes targeting c-myb, B-myb, NmU, or NMUR1 were used to silence their respective endogenous expression. We prepared c-myb siRNA as described14 using sequences 5′-UGUUAUUGCCAAGCACUUAAA-3′ and 5′-UAAGUGCUUGGCAAUAACAGAA-3′. B-myb and control siRNAs were from Dharmacon RNAi Technologies. The mature NmU and NMUR1 siRNAs were from Sigma-Aldrich. Control, c-myb, and B-myb siRNA (5 μg) were nucleofected into K562 cells (1 × 106 cells) using nucleofection kit V (Lonza) and program T-16 on the Amaxa nucleofector according to the manufacturer's instructions. Control, c-myb, NmU, or NMUR1 siRNA (1-5 μg) were nucleofected into primary CD34+ cells (1 × 106) using nucleofection kit CD34+ (Lonza) and program U-08 on the Amaxa nucleofector as per the manufacturer's instructions. The nucleofection efficiencies of siRNA into K562 and CD34+ cells were ≧ 85% and ≧ 65%, respectively (data not shown).
Quantitative real-time PCR
Total RNA was isolated from K562 and CD34+ cells using RNeasy Mini kit with on-column DNase I digestion (QIAGEN) as per the manufacturer's instructions. The purified RNA was reverse transcribed as described.14 When c-myb or B-myb expression was silenced in K562 cells, the resultant cDNA was amplified in the TaqMan Universal Master Mix in the presence of gene-specific primers (0.2μM) and the appropriate gene-specific TaqMan probe (0.1μM) in an iCycler (Bio-Rad). When RNA was isolated from CD34+ cells cultured under erythroid-inducing conditions, the resultant cDNA was amplified using SYBR Green PCR Master Mix (Applied Biosystems) with forward and reverse gene-specific primers (0.2μM) in an iCycler (Bio-Rad). The sequences of the forward and reverse primers and the TaqMan probes used to amplify c-myb, NmU, and 18S were as described.14 The sequences of the forward and reverse primers and the TaqMan probe used to amplify B-myb were as described.28 The forward and reverse primer sequences used to amplify NMUR1 were (5′-GGCTCCAGCAGCACGATc-3′) and (5′-GCAGATGCCAAACACCACG-3′), respectively. Expression levels of c-myb, B-myb, NmU, and NMUR1 were normalized to 18S.
Immunofluorescence microscopy
K562 as well as non-nucleofected or nucleofected CD34+ cells (at least 2 × 104) were cultured overnight in serum-free media, then added to fibronectin-coated CultureWell chambered coverglass (Grace Bio-Labs) and unstimulated or stimulated with NmU (17μM; Invitrogen) for 30 minutes at 37°C. After a 30-minute incubation with NmU, the cells were washed with phosphate-buffered saline, fixed in 1% paraformaldehyde for 20 minutes at room temperature, and permeabilized with 0.1% Triton X-100 for 5 minutes at room temperature. Then, cells were first incubated with Image It signal enhancer (Invitrogen), second with human PKC-βII antibody (c-18; Santa Cruz Biotechnology), followed by AlexaFluor-488 goat anti–rabbit IgG (H + L; Invitrogen) as recommended by the manufacturers. The washed cells were finally counterstained with Prolong-Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) and imaged using an Olympus BX60 upright microscope equipped with Openlab software (Improvision; original magnification ×400).
Generation of NmU luciferase reporter constructs
Genomic DNA was isolated from K562 cells using the QIAamp DNA Mini Kit (QIAGEN) as directed by the manufacturer. The GC-RICH PCR System (Roche Applied Science) was used to amplify a 2000-bp region of the human NmU gene. Briefly, genomic DNA (350 ng), forward (5′-ACATTTCACTCTCACACTGGCTGc-3′) and reverse (5′-AGAGAAGCCAAGTGAAGTCCCAGA-3′) primers (200nM each primer), 200nM dNTPs, 2.5 μL of the Gc-rich resolution solution, and 1 times GC-RICH PCR buffer were combined with the enzyme mix and amplified for 35 cycles as per the manufacturer's instructions. The resultant PCR product was subcloned into pCR-XL-TOPO vector using the TOPO XL PCR Cloning Kit (Invitrogen) according to the manufacturer's instructions to yield pCR-NmU. The NmU promoter sequence in pCR-NmU was removed by digestion with Mlu I (New England Biolabs) and XhoI (New England Biolabs) and then ligated using T4 DNA ligase (Promega) into the Mlu I and XhoI sites upstream of the firefly luciferase reporter gene in pGL3-basic (Promega) to yield pGL3-NmU. Deletion of Myb response element (MRE) 6 through 11 (del MRE6-11) in the NmU promoter was accomplished by standard PCR protocols using pGL3-NmU as the template and the forward (5′-ATAACCAGCGCTCCAGACCAGTTA-3′) and reverse (5′-AGAGAAGCCAAGTGAAGTCCCAGA-3′) primers. The resultant PCR product was subcloned into pCR-2.1 using the TOPO PCR Cloning Kit (Invitrogen) as per the manufacturer's instructions. The truncated NmU promoter in pCR-2.1 was digested with Kpn I and XhoI and inserted into the Kpn I and XhoI sites in pGL3-basic. Individual deletion of MRE9 (del MRE9) and MRE10 (del MRE10) in the NmU promoter was completed using mutagenic primer sequences for del MRE9-forward primer (5′-CCTTTCTTACATGCTTCCTGTGTATAAAAAAGAAGGTGGCCATTATTTTTTTT-3′) and reverse primer (5′-AAAAAAAATAATGGCCACCTTCTTTTTTATACACAGGAAGCATGTAAGAAAGG-3′) and del MRE10-forward primer (5′-AATCCAGTATTTTCTCACCCATCCTTGGACCAGACGCTAA-3′) and reverse primer (5′-TTAGCGTCTGGTCCAAGGATGGGTGAGAAAATACTGGATT-3′) and the QuikChange site-directed mutagenesis kit (Stratagene) as directed by the manufacturer. The sequences of full-length and mutated human NmU promoters in pGL3-NmU were confirmed by DNA sequence analyses.
Dual-luciferase reporter assays
K562-MERT cells (1 × 106) in log-phase growth were nucleofected using kit V (Lonza) and program T-16 with pGL3-NmU or pGL3-NmU mutant promoter constructs (2 μg) and phRL-CMV (Promega) (0.028 μg), which expresses Renilla luciferase and was used to normalize transfection efficiencies as per the manufacturer's instructions. CD34+ cells (2 × 106) were nucleofected using a CD34+ kit (Lonza) and program U-08 with pGL3-NmU or mutant promoter constructs (10 μg) and phRL-CMV (Promega; 2 μg) in the absence and presence of pcDNA3-c-myb expression construct (10 μg). After culturing K562-MERT cells in the absence or presence of tamoxifen or CD34+ cells in IL-3 (10 ng/mL), IL-6 (10 ng/mL), and SCF (25 ng/mL) 48 hours after nucleofection, the cells were lysed and luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega) and a luminometer (Monolight 2010; Analytical Luminescence Laboratory) according to the manufacturer's instructions.
ChIP assays
Chromatin immunoprecipitation (ChIP) assays were completed as described29 with the following modifications. The DNA-protein cross-linking was performed in a final concentration of 0.4% formaldehyde at 37°C for 15 minutes with gentle agitation. After quenching the cross-linking reaction with glycine (0.125M), the cells were lysed in cell lysis buffer (10 minutes; 4°C). The nuclei recovered from the lysed cells were incubated in nuclei lysis buffer (10 minutes, 4°C), followed by sonication to shear the chromatin (8 pulses; 30 seconds/pulse at 50%-60% of maximum power) with a Branson Sonifier 450 (Branson Ultrasonics Corporation) to yield 500-bp chromatin fragments. After preclearing the soluble chromatin with preimmune serum, an aliquot of the precleared chromatin was removed (input) and used in subsequent PCR analyses. The remainder of the precleared chromatin was immunoprecipitated using anti–c-Myb (clone 1-1; Millipore), anti–B-Myb (Santa Cruz Biotechnology), or IgG (nonspecific antibody). The positive control ChIP assay was completed with anti–acetyl-histone H4, and the negative control ChIP assays included no chromatin and no antibody. After extensively washing the Protein A-agarose beads, the DNA-protein-antibody complexes were eluted twice with elution buffer (100 μL; 0.1M NaHCO3 and 1% sodium dodecyl sulfate). After reversing the cross-links and RNase A and proteinase K digestion, the DNA was isolated using a PCR Purification Kit (QIAGEN) per the manufacturer's instructions. To determine the interaction of c-Myb or B-Myb with NmU's promoter region in vivo, the purified DNA (7 μL) was combined with SYBR Green PCR Master Mix (Applied Biosystems) and forward and reverse primers (0.5 mM each) that flank MRE1, MRE2-5, MRE6, MRE7-8, and MRE9-11. In addition, cyclin B1 specific primers as described28 were used to amplify the DNA recovered from the anti–B-Myb ChIP assays. Primer sequences used to amplify MREs in the NmU promoter after ChIP assays are available on request.
Statistical analyses
Statistical analyses of the data were completed using the Student t test. P values < .05 were considered statistically significant for all analyses.
Results
NmU functions as a cofactor to facilitate the formation of erythroid progenitors
Our identification of NmU as a novel candidate Myb target gene in K562 cells led us to postulate that NmU functions along the myeloid lineages. Therefore, we determined the ability of NmU to enhance erythropoiesis or myelopoiesis (Figure 1). To measure erythropoiesis, we conducted colony-forming assays to generate BFU-E and CFU-E. When primary human CD34+ cells were cultured in the presence of EPO, SCF, and NmU peptide, a 30% increase in the frequency of BFU-E was observed compared with cells cultured with EPO and SCF (Figure 1A; P < .05). Culturing CD34+ cells in the presence of EPO and NmU peptide increased the frequency of CFU-E 30% compared with cells cultured with EPO (Figure 1B; P < .05). The addition of NmU to cultures of CD34+ cells containing IL-3 and GM-CSF did not alter the frequency of CFU-GM compared with cells cultured with only IL-3 and GM-CSF (Figure 1C). Combined, these data suggest that NmU enhances the EPO effect.
NmU functions to facilitate the production of erythroid progenitors from primary human CD34+ cells. Primary human CD34+ cells isolated from cord blood were cultured in methylcellulose with (A) EPO and SCF in the absence and presence of NmU to produce BFU-E, (B) EPO without and with NmU to form CFU-E, or (C) IL-3 and GM-CSF in the absence and presence of NmU to yield CFU-GM. The frequencies of CD34+-derived BFU-E, CFU-E, and CFU-GM are presented from duplicate determinations from 3 independent experiments. *P < .05.
NmU functions to facilitate the production of erythroid progenitors from primary human CD34+ cells. Primary human CD34+ cells isolated from cord blood were cultured in methylcellulose with (A) EPO and SCF in the absence and presence of NmU to produce BFU-E, (B) EPO without and with NmU to form CFU-E, or (C) IL-3 and GM-CSF in the absence and presence of NmU to yield CFU-GM. The frequencies of CD34+-derived BFU-E, CFU-E, and CFU-GM are presented from duplicate determinations from 3 independent experiments. *P < .05.
To determine whether NmU is important for erythroid progenitor formation, we silenced NmU or NMUR1 gene expression in human primary CD34+ cells, and then performed colony-forming assays to generate CFU-GM, BFU-E, and CFU-E (Figure 2). We determined by real-time PCR that, at 24 hours after nucleofection, the endogenous expression of NmU decreased 66% in NmU siRNA-treated cells, whereas endogenous NMUR1 expression decreased 96% in NMUR1 siRNA-treated cells compared with control siRNA-treated cells (Figure 2A and E, respectively). No significant change in the CFU-GM frequency was observed between control and NmU siRNA-treated CD34+ cells (Figure 2B). However, CD34+ cells nucleofected with NmU siRNA yielded BFU-E and CFU-E frequencies that were 42% and 60%, respectively, less than the BFU-E and CFU-E produced from CD34+ cells nucleofected with control siRNA (Figure 2C and D, respectively). Exogenous addition of NmU peptide to cells nucleofected with NmU siRNA restored BFU-E to the level observed with control siRNA-treated cells and increased CFU-E 345% relative to the CFU-E observed in control siRNA-treated cells (Figure 2C and D, respectively). Culturing NmU siRNA-treated cells with exogenous neurotensin, a neuropeptide reported to not interact with NMUR1,15 did not rescue BFU-E or CFU-E. Silencing NMUR1 in CD34+ cells impaired BFU-E and CFU-E frequencies 70% and 64%, respectively, compared with control siRNA-treated cells (Figure 2F and G, respectively). Exogenous addition of NmU or neurotensin peptide to NMUR1 siRNA-treated cells did not rescue BFU-E or CFU-E. Taken together, these data indicate that NmU interacts with NMUR1 to stimulate erythropoiesis.
Exogenous NmU peptide rescues impaired BFU-E and CFU-E production from primary human CD34+ cells in which endogenous NmU is silenced. Human primary CD34+ cells nucleofected with control, NmU, or NMUR1 siRNA were cultured in methylcellulose. (A) At 24 hours after nucleofection, NmU expression was determined by quantitative real-time PCR. The data represent the mean of a triplicate determination. The frequencies of (B) CFU-GM, (C) BFU-E, and (D) CFU-E derived from control and NmU siRNA-treated CD34+ cells are presented from duplicate determinations from 2 independent experiments. White bars represent control siRNA-treated cells; black bars, NmU siRNA-treated cells; dark gray bars, NmU siRNA-treated cells cultured with exogenous NmU peptide; and light gray bars, NmU siRNA-treated cells cultured with exogenous neurotensin. (E) NMUR1 expression in CD34+ cells 24 hours after nucleofection of NMUR1 siRNA was determined by quantitative real-time PCR. The frequencies of (F) BFU-E and (G) CFU-E produced from control and NMUR1 siRNA-treated cells are presented from duplicate determinations from 3 independent experiments. The white bars represent control siRNA-treated cells; black bars, NMUR1 siRNA-treated cells; dark gray bars, NMUR1-treated cells cultured with exogenous NmU peptide; and light gray bars, NMUR1-treated cells cultured with exogenous neurotensin.
Exogenous NmU peptide rescues impaired BFU-E and CFU-E production from primary human CD34+ cells in which endogenous NmU is silenced. Human primary CD34+ cells nucleofected with control, NmU, or NMUR1 siRNA were cultured in methylcellulose. (A) At 24 hours after nucleofection, NmU expression was determined by quantitative real-time PCR. The data represent the mean of a triplicate determination. The frequencies of (B) CFU-GM, (C) BFU-E, and (D) CFU-E derived from control and NmU siRNA-treated CD34+ cells are presented from duplicate determinations from 2 independent experiments. White bars represent control siRNA-treated cells; black bars, NmU siRNA-treated cells; dark gray bars, NmU siRNA-treated cells cultured with exogenous NmU peptide; and light gray bars, NmU siRNA-treated cells cultured with exogenous neurotensin. (E) NMUR1 expression in CD34+ cells 24 hours after nucleofection of NMUR1 siRNA was determined by quantitative real-time PCR. The frequencies of (F) BFU-E and (G) CFU-E produced from control and NMUR1 siRNA-treated cells are presented from duplicate determinations from 3 independent experiments. The white bars represent control siRNA-treated cells; black bars, NMUR1 siRNA-treated cells; dark gray bars, NMUR1-treated cells cultured with exogenous NmU peptide; and light gray bars, NMUR1-treated cells cultured with exogenous neurotensin.
NmU promotes the expansion of erythroblasts derived from primary CD34+ cells
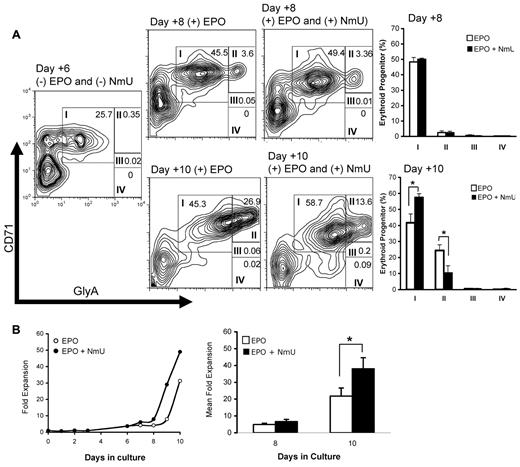
When NmU siRNA-treated CD34+ cells were cultured with EPO and exogenous NmU, the CFU-E frequency was greater than the frequency observed in control siRNA-treated CD34+ cells (Figure 2D, compare dark gray and white bars), suggesting that NmU functions to stimulate the proliferation of committed erythroid progenitors. To identify the erythroid population(s) expanded by NmU, we cultured CD34+ cells under erythroid-inducing conditions in the absence and presence of NmU peptide (Figure 3A). At day 6, before adding EPO without and with NmU peptide to the culture, early erythroid progenitors expressing high CD71 and moderate GlyA expression levels (group I) were observed (23%). At day 8, which was 2 days after adding either EPO or EPO and NmU peptide, we observed no difference in the percentages of cells in groups I through IV (Figure 3A bar graph). At day 10, which was 4 days after adding EPO or EPO and NmU peptide, we observed a 16% expansion of early erythroid progenitors in cultures with EPO and NmU compared to cultures with EPO (Figure 3A bar graph: group I, 58% vs 42%, respectively, P < .05). In addition, there was a 14% decrease in group II cells in cultures with EPO and NmU compared with EPO cultures (Figure 3A bar graph: 10% vs 24%, respectively, P = .03), although no differences in the percentages of cells in groups III and IV were observed between the 2 different culture conditions. The total number of primary cells in erythroid-inducing conditions with NmU was 2-fold more than cells in erythroid-inducing conditions without NmU at day 10 (Figure 3B, P < .05). Combined, these data strongly suggest that NmU enhances the expansion of early erythroid progenitors.
NmU enhances primary erythroid progenitor expansion. Primary human CD34+ cells from cord blood were cultured under erythroid-inducing conditions in the absence and presence of NmU peptide. (A) At days 6, 8, and 10 in culture, cells were stained for CD71 and GlyA expression, detected by flow cytometry, and analyzed by FlowJo software Version 4.5.8. Maturation-specific erythroid gates were drawn in accordance with flow cytometry models of human erythroid development.27 High expression of CD71 and moderate expression of GlyA are observed in the earliest erythroblasts (group I). As the cells mature, GlyA expression increases whereas CD71 expression declines (groups II-IV). Shown are the results from a representative flow cytometry experiment from 3 independent determinations. The plots to the right of flow cytometry experiments completed on days 8 and 10 compare the average percentage of cells in groups I through IV in the presence of EPO or EPO and NmU peptide. *P < .05. (B) CD34+ cells from cord blood cultured in the presence of IL-3, IL-6, and SCF; EPO without and with NmU was added on day 6 of culture. The cell number is presented as fold expansion relative to the CD34+ cell number on day 0. A representative fold expansion dot plot is shown from 3 independent experiments. The mean fold expansion at days 8 and 10 are shown as a bar graph. *P < .05.
NmU enhances primary erythroid progenitor expansion. Primary human CD34+ cells from cord blood were cultured under erythroid-inducing conditions in the absence and presence of NmU peptide. (A) At days 6, 8, and 10 in culture, cells were stained for CD71 and GlyA expression, detected by flow cytometry, and analyzed by FlowJo software Version 4.5.8. Maturation-specific erythroid gates were drawn in accordance with flow cytometry models of human erythroid development.27 High expression of CD71 and moderate expression of GlyA are observed in the earliest erythroblasts (group I). As the cells mature, GlyA expression increases whereas CD71 expression declines (groups II-IV). Shown are the results from a representative flow cytometry experiment from 3 independent determinations. The plots to the right of flow cytometry experiments completed on days 8 and 10 compare the average percentage of cells in groups I through IV in the presence of EPO or EPO and NmU peptide. *P < .05. (B) CD34+ cells from cord blood cultured in the presence of IL-3, IL-6, and SCF; EPO without and with NmU was added on day 6 of culture. The cell number is presented as fold expansion relative to the CD34+ cell number on day 0. A representative fold expansion dot plot is shown from 3 independent experiments. The mean fold expansion at days 8 and 10 are shown as a bar graph. *P < .05.
NmU activates PKC-βII hematopoietic cells
Because we and others have reported that NmU induces an intracellular calcium ion (Ca2+) flux14,17,25,26,30,31 and that mobilized Ca2+ can directly activate the classic PKC-βII isoform to modulate hematopoietic cell proliferation and differentiation,32,33 we determined the ability of NmU to activate PKC-βII. Using immunofluorescence microscopy, we observed PKC-βII in the cytoplasm of unstimulated K562 (Figure 4A) and CD34+ cells (Figure 4C). NmU stimulation of K562 (Figure 4B) and CD34+ cells (Figure 4D-E) resulted in the accumulation of PKC-βII at the plasma membrane, which was not observed in CD34+ cells with silenced NMUR1 expression (Figure 4F). Taken together, these data demonstrate that K562 and primary CD34+ cells express functional NMUR1 and identify a novel intracellular target of NmU.
NmU activates PKc-βII in hematopoietic cells. K562 cells were (A) unstimulated or (B) stimulated with NmU peptide for 30 minutes, and the subcellular localization of PKC-βII was detected by immunofluorescence microscopy. A representative image from 5 different fields of view for each condition is shown. Subcellular localization of PKC-βII was also determined in non-nucleofected primary CD34+ cells that were (C) unstimulated or (D) stimulated with NmU peptide for 30 minutes as well as in primary CD34+ cells that were nucleofected with (E) control or (F) NMUR1 siRNA before NmU peptide stimulation for 30 minutes. As with K562 cells, representative images are shown. PKC-βII in unstimulated control and NMUR1 siRNA-treated cells resided in the cytoplasm as shown in panel C (data not shown).
NmU activates PKc-βII in hematopoietic cells. K562 cells were (A) unstimulated or (B) stimulated with NmU peptide for 30 minutes, and the subcellular localization of PKC-βII was detected by immunofluorescence microscopy. A representative image from 5 different fields of view for each condition is shown. Subcellular localization of PKC-βII was also determined in non-nucleofected primary CD34+ cells that were (C) unstimulated or (D) stimulated with NmU peptide for 30 minutes as well as in primary CD34+ cells that were nucleofected with (E) control or (F) NMUR1 siRNA before NmU peptide stimulation for 30 minutes. As with K562 cells, representative images are shown. PKC-βII in unstimulated control and NMUR1 siRNA-treated cells resided in the cytoplasm as shown in panel C (data not shown).
c-Myb directly binds to the human NmU promoter and regulates NmU expression
Given that c-Myb is required for erythropoiesis12 and our identification of NmU as a candidate Myb target gene in microarray analyses,14 we determined the expression profiles of NmU, c-myb, and NMUR1 (Figure 5A, Figure 5B, and Figure 5C, respectively) in CD34+ cells cultured under erythroid-inducing conditions in the absence and presence of NmU peptide. During the first 6 days of culture, the primary cells were cultured with IL-3, IL-6, and SCF, and we observed a peak in NmU and c-myb expression at day 2 (Figure 5A and Figure 5B, respectively). Then, at day 6, EPO and NmU or EPO were added to the cultures. In the EPO and NmU cultures at day 8, NmU and c-myb expression increased relative to day 6. At day 10, in the EPO and NmU cultures, NmU expression decreased relative to day 8, whereas c-myb expression was restored to the level observed at day 6. NmU and c-myb expression in the EPO cultures decreased at day 8 relative to day 6, and this level of expression was sustained at day 10. NMUR1 gene expression remained unchanged during the first 6 days of culture (Figure 5C). When NmU and EPO or EPO were added at day 6, NMUR1 expression decreased 50% at day 8 compared with day 6, and its expression was sustained at that level at 10 day.
NmU and c-myb have similar expression profiles in primary CD34+ cells undergoing in vitro erythroid differentiation. CD34+ cells from cord blood were cultured in the presence of IL-3, IL-6, and SCF. At day 6, EPO was added to the cultures without and with NmU. RNA was isolated from the cells at 0, 1, 2, 3, 6, 8, and 10 days in culture and reverse transcribed to yield cDNA, which was used with gene-specific primers to amplify (A) NmU, (B) c-myb, or (C) NMUR1 and normalized to 18S. The quantitative real-time PCR data are the mean of triplicate determinations and representative of 2 independent experiments.
NmU and c-myb have similar expression profiles in primary CD34+ cells undergoing in vitro erythroid differentiation. CD34+ cells from cord blood were cultured in the presence of IL-3, IL-6, and SCF. At day 6, EPO was added to the cultures without and with NmU. RNA was isolated from the cells at 0, 1, 2, 3, 6, 8, and 10 days in culture and reverse transcribed to yield cDNA, which was used with gene-specific primers to amplify (A) NmU, (B) c-myb, or (C) NMUR1 and normalized to 18S. The quantitative real-time PCR data are the mean of triplicate determinations and representative of 2 independent experiments.
To determine the ability of c-Myb and its homolog B-Myb to bind and transactivate the previously undefined human NmU promoter, we scanned the first 2000 bp upstream of NmU's predicted transcription start site using the on-line Transcription Element Search System34 to identify potential canonical 5′PyAAC(G/T)G-3′ and noncanonical MREs. Our search yielded 11 different MREs, 4 of which were canonical (Figure 6A). Of the 4 canonical MREs, MRE3 and MRE4 are conserved between human and mouse. Of the 7 noncanonical MREs, MRE9 and MRE10 are conserved between human and mouse.
The human NmU promoter contains functional MREs distal to its transcription start site. (A) Schematic representation of the NmU promoter with the location of canonical MREs (black bars) and noncanonical MREs (gray bars). MRE3, MRE4, MRE9, and MRE10 are conserved between mouse and human, which are denoted with an asterisk above each MRE. The bent arrow represents the transcription start site for NmU. (B) Luciferase activity was determined using K562-MERT cells nucleofected with full-length NmU promoter, del MRE6–11, del MRE9, or del MRE10. A schematic of the promoter constructs with the locations of canonical and noncanonical MREs as well as MREs conserved between the human and mouse as described in panel A are provided to the left of the luciferase activity graph. The “X” in del MRE9 and del MRE10 indicates the deleted MRE. The luciferase activity obtained with each construct is presented as fold induction. The DNA recovered from ChIP assays with (C) c-Myb antibody or (D) B-Myb antibody was amplified with primers that flanked MREs throughout NmU's promoter. The amplified DNA with each primer set is presented as fold enrichment, which was calculated as the ratio between the enrichment obtained with either anti-c-Myb (black bars in C), anti-B-Myb (black bars in D), or nonspecific antibody (white bars in C-D) to that obtained without antibody. The results are the mean of triplicate determinations and are representative of at least 2 independent experiments. The asterisk indicates statistically significant difference in fold enrichment between the anti-c-Myb and nonspecific antibody (P < .05). (D) Inset is the fold enrichment observed when DNA recovered from B-Myb antibody and nonspecific antibody ChIP assays were amplified with primers specific for cyclin B1's promoter. The data are the mean of a triplicate determination for 2 independent experiments. (E) Relative gene expression of NmU, c-myb, and B-myb after the nucleofection with either control (white bars) or c-myb (black bars) siRNA. (F) Relative expression of NmU, c-myb, and B-myb after nucleofection with either control (white bars) or B-myb (black bars) siRNA. The data are the mean of duplicate determinations from 2 independent experiments.
The human NmU promoter contains functional MREs distal to its transcription start site. (A) Schematic representation of the NmU promoter with the location of canonical MREs (black bars) and noncanonical MREs (gray bars). MRE3, MRE4, MRE9, and MRE10 are conserved between mouse and human, which are denoted with an asterisk above each MRE. The bent arrow represents the transcription start site for NmU. (B) Luciferase activity was determined using K562-MERT cells nucleofected with full-length NmU promoter, del MRE6–11, del MRE9, or del MRE10. A schematic of the promoter constructs with the locations of canonical and noncanonical MREs as well as MREs conserved between the human and mouse as described in panel A are provided to the left of the luciferase activity graph. The “X” in del MRE9 and del MRE10 indicates the deleted MRE. The luciferase activity obtained with each construct is presented as fold induction. The DNA recovered from ChIP assays with (C) c-Myb antibody or (D) B-Myb antibody was amplified with primers that flanked MREs throughout NmU's promoter. The amplified DNA with each primer set is presented as fold enrichment, which was calculated as the ratio between the enrichment obtained with either anti-c-Myb (black bars in C), anti-B-Myb (black bars in D), or nonspecific antibody (white bars in C-D) to that obtained without antibody. The results are the mean of triplicate determinations and are representative of at least 2 independent experiments. The asterisk indicates statistically significant difference in fold enrichment between the anti-c-Myb and nonspecific antibody (P < .05). (D) Inset is the fold enrichment observed when DNA recovered from B-Myb antibody and nonspecific antibody ChIP assays were amplified with primers specific for cyclin B1's promoter. The data are the mean of a triplicate determination for 2 independent experiments. (E) Relative gene expression of NmU, c-myb, and B-myb after the nucleofection with either control (white bars) or c-myb (black bars) siRNA. (F) Relative expression of NmU, c-myb, and B-myb after nucleofection with either control (white bars) or B-myb (black bars) siRNA. The data are the mean of duplicate determinations from 2 independent experiments.
To experimentally validate the computational data, we conducted luciferase and ChIP assays to identify regions in the NmU promoter to which Myb binds. For both types of assays, we used K562 cells because this hematopoietic cell line has been previously used to study cellular and molecular events during human erythroid development.35-40 For the luciferase assays, we used K562-MERT cells, which express a tamoxifen-regulated dominant negative c-Myb construct as previously described. The addition of tamoxifen leads to induction of the dominant negative activity and decreased endogenous c-Myb transcriptional activity. As expected, K562-MERT cells nucleofected with the full-length NmU promoter luciferase reporter construct resulted in a 2.3-fold increase in luciferase activity in the absence of tamoxifen (Figure 6B white bar). When c-Myb activity is repressed by addition of tamoxifen, luciferase activity is decreased consistent with regulation of NmU by c-Myb. To determine which of the 11 predicted MREs in NmU's promoter bound Myb, we prepared a series of mutant NmU promoter luciferase reporter constructs (Figure 6; and data not shown). Nucleofection of del 6-11 into K562-MERT cells resulted in very little luciferase activity compared with the luciferase activity observed with the full-length NmU promoter, suggesting that the functional MRE site(s) are located distal to NmU's transcription start site. Because MRE9 and MRE10 motifs are conserved between human and mouse, we individually deleted them to yield del MRE9 and del MRE10, respectively. K562-MERT cells nucleofected with either del MRE9 or del MRE10 and cultured in the absence or presence of tamoxifen yielded very little luciferase activity compared with the full-length NmU promoter construct, strongly suggesting that both sites are functional MREs in the human NmU promoter.
To confirm the in vitro luciferase assays, we performed in vivo ChIP assays using K562 cells. Amplification of DNA recovered from ChIP assays completed with c-Myb antibody revealed a significant enrichment of the DNA containing MRE9 through MRE11 (P < .05) relative to nonspecific antibody (Figure 6C). No significant difference between anti-c-Myb ChIP and the nonspecific antibody ChIP was observed at MRE1 through MRE8. Because K562 cells express c-Myb and its homolog B-Myb and because they recognize the same MRE, we determined the in vivo interaction of both proteins with NmU's promoter.13,28 When the ChIP was completed with the B-Myb antibody, no change in enrichment was observed relative to the nonspecific antibody using primers that flanked MREs within NmU's promoter (Figure 6D). Even though we did not observe enrichment of the NmU promoter after B-Myb ChIP, we did observe enrichment of the cyclin B1 promoter (Figure 6D inset), which is in good agreement with our previous report that cyclin B1 is a B-Myb target gene in K562 cells.28 Combined, these data demonstrate that c-Myb not B-Myb in hematopoietic cells directly binds to NmU's promoter.
Finally, we silenced endogenous expression of c-myb and B-myb in hematopoietic cells and measured NmU, c-myb, and B-myb expression by real-time PCR (Figure 6E and Figure 6F, respectively). The endogenous c-myb and NmU expression decreased 95% and 83%, respectively, in c-myb siRNA-treated cells 48 hours after nucleofection compared with the control siRNA-treated cells (Figure 6E). B-myb expression was unchanged between control and c-myb siRNA-treated cells. When B-myb expression was silenced by B-myb siRNA, we observed a 97% and 56% decrease in B-myb and c-myb expression, respectively, compared with control (Figure 6F). There was no change in NmU gene expression between the control and B-myb siRNA-treated cells. The diminished expression of c-myb in B-myb siRNA-treated K562 cells could be attributed to the ability of B-Myb to transcriptionally regulate c-myb expression. However, the data suggest that the c-myb expressed 48 hours after B-myb siRNA nucleofection is at a threshold level that induces NmU expression to the same extent as control-treated cells. The ChIP data along with the loss-of-expression results strongly suggest that c-Myb, not its homolog B-Myb, directly regulates NmU transcription in hematopoietic cells.
NmU rescues impaired erythroid colony formation from CD34+ cells with silenced c-myb expression
Given that c-Myb plays a critical role during erythropoiesis through the transactivation of its target genes, we hypothesized that c-Myb transcriptionally activates NmU in normal hematopoietic cells to promote erythroid progenitor production. To test our hypothesis, we performed luciferase and colony-forming assays (Figure 7). Because we determined that c-Myb regulates NmU expression in K562 cells, we determined the ability of c-Myb to bind and transactivate the NmU promoter in primary cells (Figure 7A). In the presence of c-Myb, luciferase activity was induced 3.1-fold with the full-length NmU promoter luciferase reporter construct in primary cells compared with the luciferase activity observed from the full-length NmU promoter in the absence of c-Myb. Luciferase activities from del MRE9 and del MRE10 were dramatically reduced in primary cells in the presence of c-Myb compared with luciferase activity observed with full-length NmU promoter in the presence of c-Myb, which is consistent with our luciferase assay results in K562-MERT cells (Figure 6B).
NmU rescues erythroid colony formation when endogenous c-myb expression is silenced in primary human CD34+ cells. (A) Primary human CD34+ cells were cultured with IL-3, IL-6, and SCF for 8 days and nucleofected with either full-length NmU promoter or mutant NmU promoter constructs in the absence or presence of c-myb expression construct. After 48 hours, luciferase activity was measured and presented as mean fold induction of a duplicate determination. (B) Primary human CD34+ cells were nucleofected with control or c-myb siRNA; and 48 hours after nucleofection, the expression of c-myb was determined by quantitative real-time PCR in control and c-myb siRNA-treated cells. The frequencies of (C) BFU-E and (D) CFU-E derived from CD34+ cells are presented as the mean from duplicate determinations from 3 independent experiments. White bars represent control siRNA-treated cells; black bars, c-myb siRNA-treated cells; dark gray bars, c-myb siRNA-treated cells cultured with exogenous NmU peptide; and light gray bars, c-myb siRNA-treated cells cultured with exogenous neurotensin.
NmU rescues erythroid colony formation when endogenous c-myb expression is silenced in primary human CD34+ cells. (A) Primary human CD34+ cells were cultured with IL-3, IL-6, and SCF for 8 days and nucleofected with either full-length NmU promoter or mutant NmU promoter constructs in the absence or presence of c-myb expression construct. After 48 hours, luciferase activity was measured and presented as mean fold induction of a duplicate determination. (B) Primary human CD34+ cells were nucleofected with control or c-myb siRNA; and 48 hours after nucleofection, the expression of c-myb was determined by quantitative real-time PCR in control and c-myb siRNA-treated cells. The frequencies of (C) BFU-E and (D) CFU-E derived from CD34+ cells are presented as the mean from duplicate determinations from 3 independent experiments. White bars represent control siRNA-treated cells; black bars, c-myb siRNA-treated cells; dark gray bars, c-myb siRNA-treated cells cultured with exogenous NmU peptide; and light gray bars, c-myb siRNA-treated cells cultured with exogenous neurotensin.
Next, we silenced c-myb expression in CD34+ cells and performed colony-forming assays. At 48 hours after nucleofection of c-myb siRNA into CD34+ cells, we observed a 70% decrease in c-myb expression compared with control siRNA-treated cells (Figure 7B). The frequencies of BFU-E and CFU-E from c-myb siRNA-treated cells decreased 51% and 53%, respectively, compared with control siRNA-treated cells (Figure 7C and Figure 7D, respectively), which is in agreement with previous studies.36,41 When exogenous NmU peptide was added to cultures containing c-myb siRNA-treated cells, BFU-E and CFU-E frequencies were rescued. Specifically, the BFU-E frequency from c-myb siRNA-treated cells cultured in the presence of NmU was rescued 67% compared with control siRNA-treated cells (Figure 7C). The CFU-E frequency from c-myb siRNA-treated cells cultured in the presence of NmU was not only restored to the level observed with control siRNA-treated cells, but a 75% increase in CFU-E frequency was observed compared with control siRNA-treated cells (Figure 7D). Exogenous addition of neurotensin to c-myb siRNA-treated cells did not rescue BFU-E or CFU-E. Collectively, these data provide strong evidence that c-Myb binds to and transactivates the NmU promoter in primary cells and that NmU functions during the early stages of erythropoiesis.
Discussion
c-Myb is an obligate transcription factor during erythropoiesis.12 As a result, the cellular and molecular mechanisms by which c-Myb functions during erythroid development are of great interest. In this study, we determine that the novel c-Myb target gene NmU is important during the early stages of erythroid development and that NmU in combination with EPO expands normal early erythroid progenitors. As discussed within this section, there are different possible models for how this may occur.
Because mice that are homozygous for the NmU null allele are viable with no reported anemia,21,42 it was unexpected to us that silencing NmU or NMUR1 by RNA interference in primary human CD34+ cells would impair BFU-E and CFU-E production compared with control siRNA-treated cells (Figures 2 and 7, compare black and white bars). The ability of exogenous NmU to rescue BFU-E and yield a greater number of CFU-E in NmU or c-myb siRNA-treated cells compared with control siRNA-treated cells (Figures 2 and 7), and NmU's ability to specifically expand early erythroblasts in liquid culture (Figure 3A) indicate the NmU can play an important function during the proliferative phase of erythropoiesis. Taken together, these results may suggest that NmU's effects on erythropoiesis may be limited to situations of stress erythropoiesis.
Dexamethasone, EPO, and SCF are extrinsic factors that are added in combination to erythroid progenitor cultures to replicate stress erythropoiesis. Dexamethasone, in the presence of EPO, enhances the proliferative and clonogenic activity of erythroid progenitors from mononuclear cells of human cord blood.43 Furthermore, dexamethasone-treated erythroid progenitor cells have elevated c-myb and c-kit, a c-Myb target gene, mRNA compared with cells treated with the dexamethasone antagonist ZK112,993.43,44 Here, we observed an increase in c-myb and NmU gene expression at day 8 in erythroid-inducing cultures containing NmU compared with cultures without NmU (Figure 5), suggesting that the extrinsic factor NmU either directly or indirectly activates the transcription of c-myb in erythroid progenitors. Because dexamethasone and NmU increase c-myb expression, we hypothesize that NmU plays an important role during stress erythropoiesis possibly by inducing the association of NMUR1 and EPOR to augment the activation of EPOR by EPO. Ongoing experiments are testing this hypothesis.
Currently, the mechanism whereby NmU enhances erythropoiesis is unclear. Regulation of the proliferative phase of erythropoiesis is considered to occur largely through EPO signaling. During this phase, EPO interacts with EPOR to induce EPOR dimerization.45 Next, JAK2 is phosphorylated, which is the rate-limiting step in the process, followed by the activation of a number of downstream signaling pathways such as the mitogen-activated protein kinase pathway.46-48 In light of NmU's ability to activate extracellular signal-regulated kinase 1/2, the terminal component in the mitogen-activated protein kinase pathway, in nonhematopoietic cells,26,31 and our results here, which indicate that NmU functions to enhance the EPO effect, one possibility is that NmU binds to NMUR1 to sensitize the cells to EPO and increase proliferation of erythroid progenitors. We are currently testing this possibility.
Extrinsic factors that activate PKC-βII, either by changes in its subcellular localization or its expression, have been implicated in the proliferation-differentiation of hematopoietic cells.33,49 For instance, treating K562 cells with phorbol myristate acetate decreased endogenous PKC-βII levels and resulted in megakaryocytic differentiation.49 Subsequent removal of phorbol myristate acetate from the K562 cell cultures increased PKC-βII expression that in turn increased cell proliferation. Because PKC-βII activation depends on the binding of Ca2+, a secondary messenger that is mobilized by EPO during erythropoiesis and by NmU,14,25,26,31 we determined the ability of NmU to activate PKC-βII (Figure 4). When we treated K562 and primary human CD34+ cells, which express PKC-βII,49,50 with NmU, we observed PKC-βII activation except when NMUR1 was silenced (Figure 3), strongly indicating that NmU-mediated expansion of erythroblasts involves the activation of PKC-βII. Future experiments will more clearly define how NmU-dependent signaling interacts with EPO signaling to enhance erythropoiesis.
The temporal regulation of c-myb and NmU in the presence or absence of NmU is interesting and suggests an alternative model. In the absence of NmU, the addition of EPO at day 6 of culture has no effect on c-myb mRNA because it continues to decrease at days 8 and 10. Similarly, NmU mRNA decreases (Figure 5A-B). However, when EPO and NmU are added at day 6 of culture, there is an increase in c-myb expression (Figure 5B) and an increase in NmU mRNA expression. The latter up-regulation is probably a response to the increase in c-Myb expression. This suggests that c-Myb and NmU may positively regulate each other, and the combined effect of NmU stimulation is to increase c-Myb expression and delay terminal erythroid maturation. This model will be tested in further experiments.
In conclusion, the observations in this study reveal the relevance of NmU during erythropoiesis. The role of NmU appears to be both self-perpetuating and developmentally restricted. Further avenues of investigation include determining how NmU cooperates with EPO and whether NmU can substitute for EPO as well as the role of NmU in disordered erythropoiesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cezary Swider for isolation of CD34+ cells, Joseph Conlon for assistance with conducting the ChIP experiments, Shally Iyer for assistance with immunofluorescence microscopy assays, Anna Kalota for advice on the differentiation experiments using primary cells as well as Anne Brignier, Martin Carroll, Elizabeth Hexner, Anna Kalota, and Alexander Perl for helpful discussions and critical reading of the prepared manuscript.
This work was supported by the National Institutes of Health (grant T35-HL-007789; grants K01-DK-068283 and R21-HL-095037, S.E.S.), the Leadership Alliance, and the Undergraduate Research Experience in Cancer (grant 3P30CA016520–34S1).
This work is dedicated in memory of Dr Alan Gewirtz whose mentorship and training in hematopoiesis were invaluable in performing these studies and preparing the manuscript.
National Institutes of Health
Authorship
Contribution: J.E.G. conducted the luciferase reporter and ChIP assays; S.S.D. nucleofected K562 cells with control, c-myb, or B-myb siRNA, and measured the expression of c-myb, B-myb, NmU, and 18S by real-time PCR 48 hours after nucleofection; R.L. determined the fold expansion of primary cells cultured under erythroid-inducing conditions in the absence and presence of NmU peptide and helped with luciferase reporter assays in primary cells; Y.N. generated the c-myb siRNA molecules used to specifically silence c-myb in hematopoietic cells; and S.E.S. was involved with experimental design, prepared the pGL3-NmU construct, mutated MREs within the NmU promoter of pGL3-NmU, performed the remainder of the experiments in this manuscript, conducted data analyses, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan E. Shetzline, University of Pennsylvania School of Medicine, Division of Hematology/Oncology, 421 Curie Blvd, BRB II/III, Rm 713, Philadelphia, PA 19104; e-mail: shetzlin@mail.med.upenn.edu.