Abstract
Erythropoietin (Epo) has been used in the treatment of anemia resulting from numerous etiologies, including renal disease and cancer. However, its effects are controversial and the expression pattern of the Epo receptor (Epo-R) is debated. Using in vivo lineage tracing, we document that within the hematopoietic and mesenchymal lineage, expression of Epo-R is essentially restricted to erythroid lineage cells. As expected, adult mice treated with a clinically relevant dose of Epo had expanded erythropoiesis because of amplification of committed erythroid precursors. Surprisingly, we also found that Epo induced a rapid 26% loss of the trabecular bone volume and impaired B-lymphopoiesis within the bone marrow microenvironment. Despite the loss of trabecular bone, hematopoietic stem cell populations were unaffected. Inhibition of the osteoclast activity with bisphosphonate therapy blocked the Epo-induced bone loss. Intriguingly, bisphosphonate treatment also reduced the magnitude of the erythroid response to Epo. These data demonstrate a previously unrecognized in vivo regulatory network coordinating erythropoiesis, B-lymphopoiesis, and skeletal homeostasis. Importantly, these findings may be relevant to the clinical application of Epo.
Introduction
Anemia can result from benign conditions, such as acute/chronic bleeding, dietary deficiency, renal disease or hemoglobinopathies, and malignant diseases, such as myelodysplastic syndrome.1,2 In response, the body produces erythropoietin (Epo).3 Epo acts via its receptor (Epo-R) to stimulate and support increased erythropoiesis.4,5 It has been widely used clinically for treating anemia of numerous causes, including infection, inflammation, gastrointestinal diseases, and cancer.2,6 However, the use of Epo in cancer treatment is controversial because of adverse effects on survival, which have been reported in some clinical trials.7-9
Within the erythroid lineage, Epo is essential for the survival, proliferation, and differentiation of definitive progenitors. Whereas erythroid colony-forming-units (CFU-Es) are highly Epo-dependent, Epo is dispensable for later stages of erythroid development.10 Lack of Epo signaling in the mouse results in reduced primitive erythropoiesis and death at approximately embryonic day 13 (E13) because of failure of definitive erythropoiesis.11,12 Targeted disruption of Epo or its receptor Epo-R generates identical phenotypes, suggesting that there are no alternate physiologic ligands or receptors.11 In addition to its erythropoietic activity, Epo has also been proposed to act as a tissue-protective factor in tissues, such as the heart, blood vessels, and brain.13,14 However, the expression of Epo-R in nonerythroid tissues is contentious. Recently described antibody studies suggest that Epo-R is restricted to the erythroid lineage.15-18 This raises several questions regarding the effects of Epo in nonhematopoietic cell types.
We have treated wild-type mice with a clinically relevant dose of Epo19 for 10 days, which as anticipated led to an erythropoietic response, including elevated hematocrit and hemoglobin levels. Unexpectedly, Epo administration resulted in a rapid loss of trabecular bone and impaired B-cell development within the bone marrow (BM). The nonerythropoietic effects of Epo were probably indirect via the production of cytokines/soluble factors by the Epo-responsive erythroblasts because Epo-R could not be detected on either B cells or osteoblasts. These results demonstrate an in vivo regulatory nexus coordinating erythropoiesis, B-lymphopoiesis, and skeletal homeostasis, which may be of relevance to the clinical application of Epo.
Methods
Experimental mice
Nine-week-old male C57BL/6 mice (ARC) were injected intraperitoneally with either PBS control or 300 U/kg of recombinant human Epo (rhEpo; Janssen Cilag, Epoitin α) every second day for 10 days, or twice with 40 μg/kg of phenylhydrazine (PHZ; Sigma-Aldrich). All mice received intraperitoneal injections of 20 mg/kg calcein (Sigma-Aldrich) on day 4 and day 8. One cohort of mice received a single injection of PBS or zoledronic acid (ZA, 10 μg/kg; Novartis Pharma AG) subcutaneously 3 days before starting Epo or control treatment. For the PHZ experiment, mice were bled on day 0 and day 5, in addition to day 10 when all experiments were terminated. For the Epo-R tracing experiments Epo-R-Cre knock-in mice20 were crossed with B6.129 × 1-Gt(ROSA)26Sortm1(EYFP)Cos reporter mice from The Jackson Laboratory (#006148). BM from C57BL/6 mice was used for in vitro cultures. Primary calvarial osteoblasts for osteoclast cultures were harvested as previously described.21 All experiments were performed with approval of St Vincent's Health Melbourne AEC.
Cell preparations and flow cytometry analysis
Peripheral blood (PB) was analyzed on a hematologic analyzer (Sysmex KX-21N, Roche Diagnostics). Bones were flushed, spleens crushed, and single-cell suspensions prepared. Erythroid cells in the PB were lyzed before FACS analysis. Osteoblastic progenitor populations were obtained as previously described.22 Antibodies against murine CD45.1, CD45.2, Mac1, Gr1, F4/80, B220, IgM, CD43, CD19, CD4, CD8, Ter119, CD71, CD44, Sca1, c-kit, CD34, FLT3, FcγR (CD16/32), CD41, CD51, PDGFRα, CD31, either biotinylated or conjugated with FITC, phycoerythrin, phycoerythrin-Cy5, peridinin chlorophyll protein-Cy5.5, phycoerythrin-Cy7, allophycocyanin, or allophycocyanin Alexa 750 were all obtained from eBioscience. CD105 and CD150 (clone TC15–12F12.2) were from BioLegend, and biotinylated antibodies were detected with streptavidin conjugated with Alexa-405 or Qdot 605 (Invitrogen). Retic-COUNT (BD Biosciences) was used for quantitating reticulocytes. Cells were sorted on a BD FACSAria cell sorter (BD Biosciences) or analyzed on a BD LSRIIFortessa (BD Biosciences). Results were analyzed with FlowJo software Version 8.8.6 (TreeStar).
Transplantations
A total of 2 × 105 unfractionated BM cells from C57Bl/6 mice treated with PBS or Epo (CD45.2) were competitively transplanted with 2 × 105 wild-type BM cells (CD45.1/CD45.2), into lethally irradiated (5 Gy split dose 3 hours apart; total of 10 Gy) recipients (CD45.1) through intravenous injections. A total of 6 recipients per treatment were transplanted; the experiment was performed in duplicate. PB was taken at 4, 8, 12, and 16 weeks to monitor multilineage donor reconstitution.
Colony assays
A total of 50 000/mL BM or 100 000/mL spleen cells were plated in methylcellulose medium containing 50 ng/mL murine stem cell factor (PeproTech), 10 ng/mL murine interleukin-3 (PeproTech), 50 ng/mL human IL-6 (Amgen), and 3 U/mL rhEpo (Jansen-Cilag). The methylcellulose medium used for colony assays contained 1% methylcellulose (Methocel MC #64630; Fluka), Iscove modified Dulbecco medium (Invitrogen), 20% BIT serum replacement (StemCell Technologies), penicillin/streptomycin (Invitrogen), GlutaMAX (Invitrogen), and cytokines as described. Colonies were counted on day 8 and day 12. For CFU-E colonies, 50 000 BM or spleen cells were plated in methylcellulose supplemented according to M3334 from StemCell Technologies, including 3 U/mL rhEpo. CFU-Es were scored on day 2.
In vitro cultures
For B-cell cultures, 20 000 OP9 cells/mL were plated in a 24-well plate 48 hours before 10 000 sorted pre-pro B cells were added per well. B-cell cultures were performed in α-minimum essential medium (Sigma-Aldrich) with 20% fetal bovine serum (PAA Gold), penicillin/streptomycin, GlutaMAX, 5 × 10−5M 2-mercaptoethanol (Sigma-Aldrich), 5 ng/mL Fms-like tyrosine kinase 3 ligand (Flt3L; PeproTech), and 1 ng/mL mIL-7 (R&D Systems), with or without 3 U/mL or 9 U/mL of rhEpo. B-cell development was analyzed on day 5 and 10 (n = 3/treatment). For the transwell experiment, 100 000 sorted erythroblasts were added to a 0.4-μm pore size insert per well (Corning). B-cell development was analyzed on day 7 (n = 3/treatment).
Kusa4b10 cells23 were seeded at 3000 cells/cm2 in 12-well plates and differentiated into mature osteoblasts for 21 days in α-minimum essential medium media supplemented with 50 μg/mL ascorbate (Sigma-Aldrich) and 10mM β-glycerophosphate (Sigma-Aldrich), with or without 3 U/mL or 6 U/mL of rhEpo. Cells were washed, fixed, and mineralization activity was measured using Alizarin red staining (Sigma-Aldrich).
Osteoblast were seeded at 10 000 cells together with 100 000 Ficoll-enriched BM monocytes per well in 48-well plates and grown in α-minimum essential medium/10% fetal calf serum with or without erythroblasts, and with or without Epo (6 U/mL), rmOSM (50 ng/mL, R&D Systems), or recombinant mouse Receptor Activator of Nuclear factor kappa-B Ligand (rmRANKL; 30 ng/mL, PeproTech). The wells were fixed and tartrate-resistant acid phosphatase (50 mL 0.1M acetate buffer supplemented with 5 mg naphthol AS-MX phosphate 9 [Sigma-Aldrich], 1% N,N-dimethylformide [Sigma-Aldrich], 30 mg Fast Red LB Violet Salt F3381 [Sigma-Aldrich], 0.575 g sodium tartrate [Sigma-Aldrich], pH 5); positive multinucleated cells were counted on day 7.
Gene expression and ELISA
RNA isolation and cDNA synthesis were performed using the RNeasy Mini Kit (QIAGEN) and the Invitrogen Superscript III kit, respectively. RNA quantification was done using SYBR Green (Stratagene) and primers from Integrated DNA Technology. Values were normalized against β2-mercaptoethanol. For quantification of Epo levels, an ELISA was performed according to protocol on diluted plasma (Quantikine Mouse Epo Kit, R&B Systems).
Bone and vessel analysis
Micro-computed tomography (μCT) analysis was performed according to standard procedures in the secondary spongiosa of the proximal tibia using Skyscan1076 (x-ray potential 50 KVp, Kontichm).24 Tibias were wrapped in 70% EtOH-soaked gauze, cling film within a cryovial and scanned at a voxel size of 9 μm. Bones were reconstructed using NRecon (Skyscan) with smoothing of 1, ring artifact reduction of 6 and beam-hardening correction of 35%. The trabecular region of interest was manually drawn 0.55 mm from the bottom of the growth plate for 2 mm. Three-dimensional modeling and analysis of the bones were done using CTAn (Skyscan), applying the marching cube tight model and upper and lower gray threshold values of 45 and 255.
Histomorphometry was carried out according to standard procedures using the Osteomeasure system (Osteo Metrics). Tibias were fixed and embedded in methylmethacrylate, and 5μm-sections were stained with toluidine blue (Waldeck), xylenol orange (Sigma-Aldrich), or Goldeners trichrome.25
Immunohistochemistry of the vessels was performed in the secondary spongiosa on paraffin sections of tibiae. Antigen-retrieval was undertaken using 10mM ethylenediaminetetraacetic acid, pH 7.5, at 95°C for 5 minutes. After endogenous peroxidase and nonspecific protein block (3% H2O2, 10% fetal calf serum 10% normal horse serum), anti-VEGFR3 monoclonal antibody (1.25 μg/mL, eBioscience) was incubated for 1 hour at room temperature. After secondary polyclonal antibody (goat antirabbit, BD Biosciences PharMingen) and streptavidin horseradish peroxidase incubation (Dako Australia), tyramide signal amplification (PerkinElmer Life and Analytical Sciences) was used, followed again by streptavidin horseradish peroxidase incubation. Staining was developed with 3′3′-diaminobenzidine+ (Sigma-Aldrich) and briefly counterstained with Mayer hematoxylin. The perimeter of VEGFR3+ vessels were manually traced and quantified using the Osteomeasure system.
The pictures in Figures 4 and 5 were taken using an Axio Imager.A1 microscope (Zeiss), EC Plan NEOFLUAR objective lens 2.5×/0.015 NA, 10×/0.3NA, or 20×/0.5NA (no imaging medium), and an AxioCam MRc5 camera (Zeiss). The software AxioVs40 V4.8.1.0 was used to acquire the images digitally, and the brightness of some pictures was adjusted using Adobe Photoshop CS5.
Statistical analysis
The significance of results was analyzed using the unpaired 2-tailed Student t test on the bases that highly inbred genetically identical mice have been used in all experiments, P < .05 was considered significant. All data are presented as mean ± SEM.
Results
In vivo expression of Epo-R in hematopoietic and mesenchymal cells
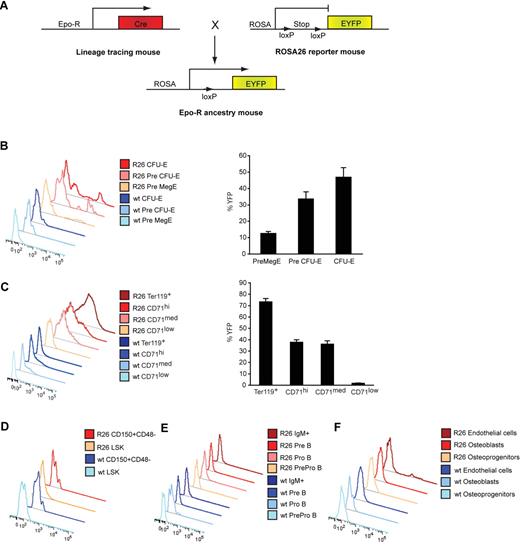
The expression pattern of Epo-R is highly controversial because of questions raised over the specificity of antibodies previously used to detect its expression. To clarify the occurrence of Epo-R in vivo independently of antibodies, we used a lineage-tracing strategy to map its expression pattern. By crossing Epo-R-Cre knock-in mice,20 where GFP-Cre is targeted to the endogenous Epo-R initiation codon, with Rosa26 yellow fluorescent protein (eYFP) reporter mice we could track the expression of Epo-R in different cell populations using FACS (Figure 1A). Using this strategy, any cell that has expressed Epo-R transcript during its development is permanently labeled eYFP+ until its death or enucleation. The intensity of eYFP is not a direct reflector of the level of Epo-R expression. The eYFP transcript is expressed from the Rosa26 promoter, and its expression acts as a surrogate marker of the transcriptional activity of the Epo-R locus. The expression of Epo-R first became detectable in pre-MegE progenitors (Figure 1B; for FACS plot, see Figure 2L)27 and became robust from the pre–CFU-E population onward. More than 70% of the Ter119+ fraction was eYFP-labeled (Figure 1C). Epo-R is known to be down-regulated as erythroblasts mature11,28,29 ; however, eYFP expression was as expected retained until the erythroid progenitors enucleated (CD71low population; Figure 1C; for FACS plot, see Figure 2E). Based on eYFP labeling, there was no Epo-R expression in the highly purified LKS+CD150+CD48− hematopoietic stem cells (HSCs), B-lymphoid or myeloid lineages (Figure 1D-E; data not shown; for mean fluorescent intensity, see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For FACS plots, see Figure 3A and Figure 6A. The lack of myeloid Epo-R expression is consistent with the analysis of the expression of Epo-R transcript at a single-cell level performed by Pronk et al.27 Analysis of mesenchymal and osteoblastic-enriched populations from the bone microenvironment22,26 revealed that these niche cells do not express Epo-R (Figure 1F). However, a low percentage of the endothelial-enriched fraction expressed eYFP (Figure 1F), consistent with previously published data.15 Thus, in vivo Epo-R expression within the BM microenvironment is essentially restricted to the erythroid population.
In vivo expression of Epo-R in hematopoietic and mesenchymal cells. (A) In vivo lineage-tracing model to map the expression pattern of the Epo-R. Epo-R-Cre knock-in mice20 were crossed with Rosa26 yellow fluorescent protein (eYFP) reporter mice, resulting in eYFP labeling of cells, which has expressed Epo-R at any time of its development. FACS analysis of eYFP expression in (B) early erythroid progenitors depicting a representative plot (left) and quantification (right), (C) mature erythroid progenitors, and total Ter119+ cells showing a representative plot (left) and quantification (right), and representative plots of (D) HSCs, (E) B-cell progenitors, and (F) osteoblastic and endothelial cell fractions.22,26 Data are presented as mean ± SEM; n = 4.
In vivo expression of Epo-R in hematopoietic and mesenchymal cells. (A) In vivo lineage-tracing model to map the expression pattern of the Epo-R. Epo-R-Cre knock-in mice20 were crossed with Rosa26 yellow fluorescent protein (eYFP) reporter mice, resulting in eYFP labeling of cells, which has expressed Epo-R at any time of its development. FACS analysis of eYFP expression in (B) early erythroid progenitors depicting a representative plot (left) and quantification (right), (C) mature erythroid progenitors, and total Ter119+ cells showing a representative plot (left) and quantification (right), and representative plots of (D) HSCs, (E) B-cell progenitors, and (F) osteoblastic and endothelial cell fractions.22,26 Data are presented as mean ± SEM; n = 4.
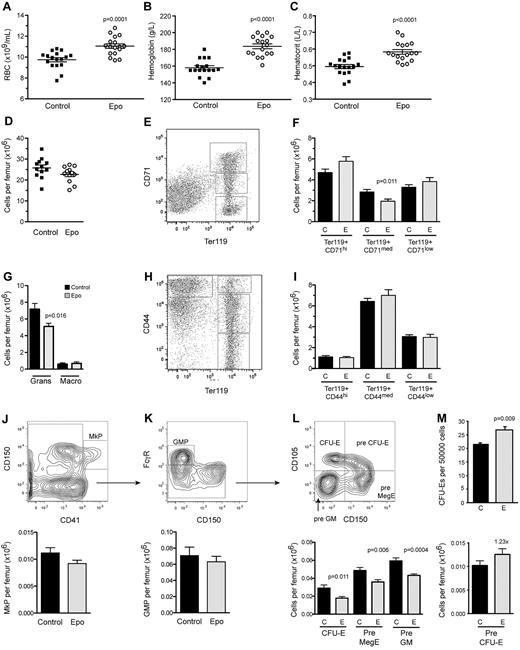
Administration of a low dose of Epo induces a mild increase in peripheral erythropoiesis, whereas marrow erythropoiesis remains largely unaffected. Differential counts of (A) RBCs, (B) hemoglobin, and (C) hematocrit in PB 10 days after the first PBS or Epo injection (n = 17 per treatment). (D) Total BM cellularity. Fractionation of erythroid differentiation in BM using (E-F) CD71 and Ter119 or (H-I) CD44 and Ter119. The Ter119+/CD71hi and Ter119+/CD44hi fractions represent the proerythroblasts and basophilic erythroblasts, Ter119+/CD71med and Ter119+/CD44med compose the polychromatic and orthochromatic erythroblasts, and Ter119+/CD71low and Ter119+/CD44low contain enucleated reticulocytes and mature erythrocytes. (G) Numbers of granulocytes and macrophages, as determined by FACS using the markers Mac1, Gr1, and F4/80. Further fractionation of myeloid and erythroid progenitor subsets by FACS into (J) megakaryocyte progenitors (MkP), (K) myeloid progenitors (granulocyte macrophage progenitor), and (L) CFU-Es, Pre MegE, and Pre GM (left) and Pre CFU-E progenitors (right). (M) BM CFU-E colonies (n = 5). BM was collected 10 days after the first PBS or Epo injection (n = 12 per treatment if not stated otherwise). All data are presented as mean ± SEM.
Administration of a low dose of Epo induces a mild increase in peripheral erythropoiesis, whereas marrow erythropoiesis remains largely unaffected. Differential counts of (A) RBCs, (B) hemoglobin, and (C) hematocrit in PB 10 days after the first PBS or Epo injection (n = 17 per treatment). (D) Total BM cellularity. Fractionation of erythroid differentiation in BM using (E-F) CD71 and Ter119 or (H-I) CD44 and Ter119. The Ter119+/CD71hi and Ter119+/CD44hi fractions represent the proerythroblasts and basophilic erythroblasts, Ter119+/CD71med and Ter119+/CD44med compose the polychromatic and orthochromatic erythroblasts, and Ter119+/CD71low and Ter119+/CD44low contain enucleated reticulocytes and mature erythrocytes. (G) Numbers of granulocytes and macrophages, as determined by FACS using the markers Mac1, Gr1, and F4/80. Further fractionation of myeloid and erythroid progenitor subsets by FACS into (J) megakaryocyte progenitors (MkP), (K) myeloid progenitors (granulocyte macrophage progenitor), and (L) CFU-Es, Pre MegE, and Pre GM (left) and Pre CFU-E progenitors (right). (M) BM CFU-E colonies (n = 5). BM was collected 10 days after the first PBS or Epo injection (n = 12 per treatment if not stated otherwise). All data are presented as mean ± SEM.
Epo impairs B-lymphopoiesis at the pre-pro to pro B-cell transition. (A) B-cell differentiation as analyzed by FACS using the indicated surface markers. Impaired B-cell differentiation in the BM caused by both (B-C) exogenous rhEpo and (D-E) endogenous Epo as a result of PHZ-induced hemolytic anemia. Data are mean ± SEM and were collected 10 days after the first PBS, Epo, or PHZ injection. n = 12 for Epo and 8 for PHZ treatment.
Epo impairs B-lymphopoiesis at the pre-pro to pro B-cell transition. (A) B-cell differentiation as analyzed by FACS using the indicated surface markers. Impaired B-cell differentiation in the BM caused by both (B-C) exogenous rhEpo and (D-E) endogenous Epo as a result of PHZ-induced hemolytic anemia. Data are mean ± SEM and were collected 10 days after the first PBS, Epo, or PHZ injection. n = 12 for Epo and 8 for PHZ treatment.
Administration of Epo induces increased erythropoiesis
To examine in detail the activity of Epo in light of the lineage tracing described in Figure 1, we injected 9-week-old male C57BL/6 mice every second day for 10 days with Epo (rhEpo; 300 U/kg/dose; supplemental Figure 1A). The PB red blood cell (RBC) number was significantly increased by 13% (Figure 2A), with an accompanying 16% to 18% elevation in hemoglobin and hematocrit levels in mice treated with Epo (Figure 2B-C). Circulating reticulocytes were increased 2-fold (data not shown), indicative of increased erythropoiesis, whereas PB leukocyte and platelet numbers were unaffected using this regimen (supplemental Figure 1B-C).
Total BM cellularity remained unchanged by Epo treatment (Figure 2D). Marrow erythropoiesis was largely normal as assessed by either Ter119/CD71 (Figure 2E-F),30 or Ter119/CD44 fractionation (Figure 2H-I),31 except for a slight reduction of the Ter119+/CD71med expressing erythroblasts (Figure 2F). Using a high-fidelity FACS protocol,27 we observed that the major effect of Epo on erythropoiesis occurred with a 60% expansion of the pre–CFU-E phenotypic fraction (23% increase of total number/femur; Figure 2L right), which correlated well with its robust expression of the Epo-R (Figure 1B). Despite reduced numbers of phenotypic CFU-Es (Figure 2L left), Epo treatment resulted in a 25% increase in CFU-E colonies when BM cells from control and Epo-treated mice were plated in methylcellulose (Figure 2M).
The expanded erythroid compartment was largely supported by extramedullary erythropoiesis in the spleen. Spleen weight and cellularity were increased in the Epo-treated cohorts (supplemental Figure 2A), and the splenic proerythroblasts through to orthochromatic erythroblast fractions were 2- to 3-fold increased compared with controls (supplemental Figure 2E-F).30,31 Furthermore, the number of CFU-E colonies was highly increased in spleens of Epo-treated mice compared with control mice (supplemental Figure 2G). Thus, Epo induces expansion of the pre–CFU-E fraction in the BM and extramedullary erythropoiesis in the spleen.
Epo treatment impairs B-cell development
Within the BM, there were changes in cell populations that do not express Epo-R. The number of granulocytes in the BM was decreased (Figure 2G). This was accompanied by a significant reduction of PreGM progenitors (Figure 2L), although no difference was detected in the granulocyte macrophage progenitor fraction (Figure 2K). The megakaryocyte progenitor fraction remained unchanged (Figure 2J), consistent with the unaltered platelet numbers in PB.
Intriguingly, we observed a rapid alteration in the maturation of the B-lymphoid lineage (Figure 3A). Total B-lymphopoiesis in the BM was compromised by Epo treatment, with reductions in both the IgM+ and the more immature fractions (Figure 3B). More detailed fractionation revealed impairment of the pro B-cell and pre B-cell stages (28% and 40% reduction, respectively; Figure 3C), whereas the number of pre-pro B cells, the most primitive committed population assessed, was unaffected. Consistent with decreased B-lymphopoiesis, reduced expression levels of Il-7 and Cxcl12 transcripts were observed in unfractionated BM isolates from mice treated with Epo (supplemental Figure 3A), whereas the expression of their respective receptors Il7r and Cxcr4 remained unchanged (data not shown). Epo was not able to directly act on the B cells as they lack Epo-R expression. In a range of coculture experiments using purified erythroblasts, pre-pro B cells and OP9 stroma, neither 3 U/mL nor 9 U/mL of Epo had any effect on B-cell development in vitro (supplemental Figure 3B; data not shown). Because it is known that osteolineage cells are essential to support normal B-lymphopoiesis,32,33 we investigated whether there were changes within the BM microenvironment that could account for the impaired B-cell maturation.
Epo induces a rapid loss of bone and increased bone turnover
We assessed bone parameters by analyzing tibiae from Epo- or PBS-treated mice using μCT (Figure 4A; supplemental Videos 1, 2) and histomorphometry (Figure 4B-K). Quantitative histomorphometry revealed that Epo treatment resulted in a 26% reduction in trabecular bone volume (Figure 4C) and a 20% decrease in trabecular number (Figure 4D). Trabecular spacing increased (Figure 4E), whereas trabecular thickness remained unchanged (Figure 4F). Furthermore, Epo treatment resulted in increased osteoclast numbers and surface per bone surface (Figure 4G-H), which was accompanied by an increase of osteoblasts (Figure 4I-J). This phenotype is indicative of an increased rate of bone turnover. The osteoid volume per bone volume was significantly increased in Epo-treated mice (Figure 4K), whereas the rate of mineralized bone formation remained unchanged (Figure 4L). The single calcein-labeled surface was considerably decreased (Figure 4M), whereas the double-labeled surface remained unchanged (Figure 4N), indicating increased osteoclast activity. To further investigate the underlying mechanism, we performed a number of ex vivo and in vitro experiments. We could not detect changes in the concentration of circulating parathyroid hormone or in the expression levels of osteoclast modulating cytokines, such as RANKL and OPG in unfractionated BM from mice treated with Epo (data not shown). Furthermore, we could not detect any support of osteoclast differentiation when coculturing primary calvarial osteoblasts and mononuclear BM cells in the presence of Epo, as measured by tartrate-resistant acid phosphatase staining (data not shown). We also investigated the effects of Epo on osteoblastic cells by differentiating the osteoprogenitor cell line Kusa4b10 in the presence or absence of Epo. After measuring the mineralization potential at different time points using alizarin red staining, we could not detect any difference in osteoblastic function in direct response to Epo (supplemental Figure 3C). In conclusion, Epo caused a rapid and specific increase in the rate of bone turnover and a net loss in trabecular bone, which was indirect because of the lack of detectable Epo-R expression on osteolineage cells.
Epo treatment results in a substantial decrease in trabecular bone and increased bone remodeling. (A) Three-dimensional μCT analysis of the secondary spongiosa of proximal tibia depicting a representative top view (top) and side view (bottom) from PBS- and Epo-treated mice, respectively (n = 12 per treatment). (B) Representative sections (original magnification ×25) of 2-dimensional histomorphometric analysis of the same tibial region as in panel A on fixed, plastic-embedded 5-μm sections, stained with (B) Goldeners trichrome, (C-K) toluidine blue, or (L-M) xylenol orange. The box represents the region measured for histomorphometry. Histologic analysis quantification of (C) percentage of bone volume per total volume (BV/TV), (D) trabecular number (Tb.N), (E) trabecular separation (Tb.Sp), and (F) trabecular thickness (Tb.Th). Further quantification of the (G) numbers of osteoclasts per bone perimeter (N.Oc/B.Pm), (H) percentage of bone surface occupied by osteoclasts (Oc.S/BS), (I) numbers of osteoblasts per bone perimeter (N.Ob/B.Pm), and (J) percentage of bone surface occupied by osteoblasts (Ob.S/BS). (K) Quantification of the percentage of unmineralized osteoid volume per bone volume (n = 11 treatment). (L-M) Double-fluorochrome labeling with calcein depicted as (L) mineral appositional rate (MAR), (M) single labeled surface per bone surface (sL.S/BS), and (N) double-labeled surface per bone surface (dL.S/BS) (n = 8 and 11 for PBS and Epo treatment, respectively). All bones were collected 10 days after the first PBS or Epo injection. Data are presented as mean ± SEM.
Epo treatment results in a substantial decrease in trabecular bone and increased bone remodeling. (A) Three-dimensional μCT analysis of the secondary spongiosa of proximal tibia depicting a representative top view (top) and side view (bottom) from PBS- and Epo-treated mice, respectively (n = 12 per treatment). (B) Representative sections (original magnification ×25) of 2-dimensional histomorphometric analysis of the same tibial region as in panel A on fixed, plastic-embedded 5-μm sections, stained with (B) Goldeners trichrome, (C-K) toluidine blue, or (L-M) xylenol orange. The box represents the region measured for histomorphometry. Histologic analysis quantification of (C) percentage of bone volume per total volume (BV/TV), (D) trabecular number (Tb.N), (E) trabecular separation (Tb.Sp), and (F) trabecular thickness (Tb.Th). Further quantification of the (G) numbers of osteoclasts per bone perimeter (N.Oc/B.Pm), (H) percentage of bone surface occupied by osteoclasts (Oc.S/BS), (I) numbers of osteoblasts per bone perimeter (N.Ob/B.Pm), and (J) percentage of bone surface occupied by osteoblasts (Ob.S/BS). (K) Quantification of the percentage of unmineralized osteoid volume per bone volume (n = 11 treatment). (L-M) Double-fluorochrome labeling with calcein depicted as (L) mineral appositional rate (MAR), (M) single labeled surface per bone surface (sL.S/BS), and (N) double-labeled surface per bone surface (dL.S/BS) (n = 8 and 11 for PBS and Epo treatment, respectively). All bones were collected 10 days after the first PBS or Epo injection. Data are presented as mean ± SEM.
Endogenous Epo elevations result in loss of trabecular bone
To determine whether the skeletal and B-cell phenotype could be recapitulated by an elevation of endogenous Epo, mice were injected with PHZ (supplemental Figure 4A). PHZ treatment induced a hemolytic anemia and an endogenous Epo response. By day 5 after the first PHZ injection, both RBC and hematocrit levels in PB were reduced (supplemental Figure 4B). Plasma levels of endogenous Epo were elevated 7.5-fold, and there was a 12-fold increase in reticulocytes (supplemental Figure 4C). PB parameters had largely recovered by day 10 (supplemental Figure 4B-C), whereas the numbers of erythroblasts and enucleated erythrocytes in the BM and spleen remained elevated (supplemental Figure 4D-E). Strikingly, PHZ hemolysis resulted in impaired B-cell development with a reduction in the numbers of pre-B cells consistent with that seen when treating with exogenous Epo (Figure 3D-E). Furthermore, a similar bone phenotype to that observed after Epo administration was seen after acute hemolysis (supplemental Figure 5; supplemental Video 3). Collectively, these studies demonstrate that elevations in Epo, whether exogenous or endogenous, negatively regulate bone homeostasis and BM B-lymphopoiesis.
Epo reduces the number of vessels in the BM microenvironment
Sinusoidal blood vessels have proven to play an important part in the BM microenvironment.34 Because endothelial cells express low levels of Epo-R (Figure 1F), we also investigated whether Epo treatment affects blood vessels within the BM using immunohistochemistry for the endothelial marker vascular endothelial growth factor receptor 3 (VEGFR3).35 Surprisingly, Epo treatment resulted in fewer (Figure 5D) and somewhat enlarged vessels (Figure 5E), leading to a decrease in the total perimeter of vessels (Figure 5F). However, there was no difference in the total volume of vessels occupying the marrow space (Figure 5G).
Epo reduces the number of vessels in the BM microenvironment. (A-C) Representative sections (original magnification ×100) in the secondary spongiosa on paraffin sections of tibiae from PBS and Epo-treated mice for immunohistochemistry for the endothelial marker VEGFR3. v indicates vessel; tb, trabecular bone; and m, marrow. (D) Number of vessels per marrow area (total area − bone area). (E) Vessel area per vessel. (F) Total perimeter of vessels per marrow area. (G) Total volume of vessels per marrow area. n = 6 and 5 for PBS and Epo treatment, respectively. Data are mean ± SEM.
Epo reduces the number of vessels in the BM microenvironment. (A-C) Representative sections (original magnification ×100) in the secondary spongiosa on paraffin sections of tibiae from PBS and Epo-treated mice for immunohistochemistry for the endothelial marker VEGFR3. v indicates vessel; tb, trabecular bone; and m, marrow. (D) Number of vessels per marrow area (total area − bone area). (E) Vessel area per vessel. (F) Total perimeter of vessels per marrow area. (G) Total volume of vessels per marrow area. n = 6 and 5 for PBS and Epo treatment, respectively. Data are mean ± SEM.
HSCs are normal despite reduced trabecular bone
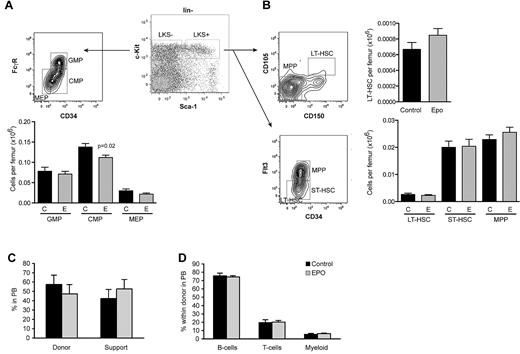
Osteolineage cells and endothelial cells are thought to constitute components of the HSC niche within the BM.34,36,37 We therefore assessed the lineage− c-Kit+ Sca1+ (LKS+) population enriched in HSCs38 and the HSC depleted LKS- fraction containing early progenitor cells. Subdivision of the LKS− fraction revealed a subtle decrease in the frequency of common myeloid progenitors after Epo treatment, whereas the granulocyte-macrophage progenitor and megakaryocyte-erythroid progenitor populations remained unchanged (Figure 6A). The LKS+ population was divided into long-term HSCs, short-term HSCs, and multipotent progenitors using 2 distinct phenotypic profiles, CD105 and CD150,27 or Fms-like tyrosine kinase 3 and CD34.39 Using either of these phentoypes, there was no apparent adverse effect on the numbers of HSCs (Figure 6B), despite the pronounced alterations in BM architecture. Despite unaltered frequency of HSCs in the BM, the loss of trabecular bone may induce mobilization. As expected, both BM and spleen cells from Epo-treated cohorts had increased numbers of erythroid colonies (supplemental Figure 6A-B). However, although the number of committed myeloid colony-forming cells was 2-fold increased in spleens from Epo-treated mice (supplemental Figure 6B,D), we did not detect increased numbers of splenic multilineage potential colonies, which would have been evidence of mobilization.40
Unaffected HSC populations in mice treated with Epo. Phenotypic analysis of hematopoietic (A) progenitor and (B) stem cell fractions by FACS using the indicated surface markers (LKS+/−; lineage− ckit+ Sca1+/−). CMP indicates common myeloid progenitor; GMP, granulocyte macrophage progenitor; MEP, megakaryocyte erythroid progenitor; LT-HSC, long-term HSCs; ST-HSC, short-term HSCs; and MPP, multipotent progenitors. n = 12 per treatment. (C) Long-term donor reconstitution and (D) lineage distribution analyzed 16 weeks after competitively transplanting 200 000 unfractionated BM cells from mice treated with either control or Epo, together with 200 000 cells from congenic wild-type mice into lethally irradiated recipients (n = 12 per treatment). Data are mean ± SEM.
Unaffected HSC populations in mice treated with Epo. Phenotypic analysis of hematopoietic (A) progenitor and (B) stem cell fractions by FACS using the indicated surface markers (LKS+/−; lineage− ckit+ Sca1+/−). CMP indicates common myeloid progenitor; GMP, granulocyte macrophage progenitor; MEP, megakaryocyte erythroid progenitor; LT-HSC, long-term HSCs; ST-HSC, short-term HSCs; and MPP, multipotent progenitors. n = 12 per treatment. (C) Long-term donor reconstitution and (D) lineage distribution analyzed 16 weeks after competitively transplanting 200 000 unfractionated BM cells from mice treated with either control or Epo, together with 200 000 cells from congenic wild-type mice into lethally irradiated recipients (n = 12 per treatment). Data are mean ± SEM.
To definitively assess HSC potential, 200 000 unfractionated BM cells from either control or Epo-treated cohorts were competitively transplanted with 200 000 BM cells from congenic wild-type mice into lethally irradiated recipients and were monitored for multilineage donor reconstitution. We did not observe any difference in long-term chimerism (Figure 6C) or lineage contribution (Figure 6D) for up to 16 weeks after transplantation. These results demonstrate that, despite the dramatic alterations within the BM microenvironment, HSC number and function were unaffected by Epo.
Bone remodeling is necessary for normal Epo response
Erythroblasts express factors, such as oncostatin M in response to Epo,3,41 which was also true in our hands (supplemental Figure 3D). Oncostatin M induces osteoblasts to secrete RANKL and thereby potentiates osteoclast maturation from monocyte/macrophage precursors.42 We hypothesized that the increased bone remodeling seen in response to Epo administration may be the result of increased secretion of factors, such as oncostatin M, by erythroblasts. This could indirectly increase osteoclast numbers and/or activity. To test this hypothesis, we cocultured primary calvarial osteoblasts and mononuclear BM cells together with Epo-responsive erythroblasts in the absence or presence of Epo. Whereas the addition of RANKL and to a lesser extent oncostatin M resulted in tartrate-resistant acid phosphatase+ multinucleated osteoclasts, Epo treatment was not capable of making the erythroblasts produce sufficient oncostatin M in vitro to induce osteoclast formation (data not shown). To investigate the involvement of osteoclasts in the described Epo-induced phenotype in vivo, we treated mice with a single dose of the bisphosphonate ZA, which inhibits osteoclast formation, function, and survival,43 and then treated them with PBS or Epo as described. ZA completely blocked the Epo-induced decrease in bone (Figure 7A; supplemental Video 4). Treatment with ZA/Epo also decreased the numbers of leukocytes per femur (Figure 7B) with an accompanying trend toward increased spleen size compared with Epo alone (Figure 7C). Surprisingly, B cells in mice treated with ZA/Epo were more severely reduced than with Epo alone (Figure 7D), clearly demonstrating that the defect in B-cell development seen when treating with Epo was not coupled to the alterations in bone.
Inhibition of osteoclast function abolishes Epo-induced bone remodeling and blunts the erythroid response to Epo. (A) Percentage of bone volume per total volume (BV/TV) as measured by μCT analysis of tibias from mice treated with PBS (P) or osteoclast-inhibiting ZA (Z) before PBS and Epo (E) treatment, respectively. (B) Number of cells per femur and (C) spleen weight of the same mice as in panel A. (D) B-lymphopoiesis as analyzed by FACS. (E-H) Quantification of the peripheral (E) RBC, (F) hemoglobin, (G) hematocrit, and (H) mean cellular volume (MCV), in ZA-treated mice. Data are mean ± SEM; n = 5 per treatment. (I) A mechanistic figure illustrating that: (1) Epo directly stimulates early erythroblasts in the BM to induce an erythroid response; (2) Epo treatment indirectly results in an osteoclast-dependent decrease of trabecular bone and increased bone remodeling; and (3) Epo treatment impairs B-cell development through unknown actions of Epo-responsive erythroblast in the BM microenvironment.
Inhibition of osteoclast function abolishes Epo-induced bone remodeling and blunts the erythroid response to Epo. (A) Percentage of bone volume per total volume (BV/TV) as measured by μCT analysis of tibias from mice treated with PBS (P) or osteoclast-inhibiting ZA (Z) before PBS and Epo (E) treatment, respectively. (B) Number of cells per femur and (C) spleen weight of the same mice as in panel A. (D) B-lymphopoiesis as analyzed by FACS. (E-H) Quantification of the peripheral (E) RBC, (F) hemoglobin, (G) hematocrit, and (H) mean cellular volume (MCV), in ZA-treated mice. Data are mean ± SEM; n = 5 per treatment. (I) A mechanistic figure illustrating that: (1) Epo directly stimulates early erythroblasts in the BM to induce an erythroid response; (2) Epo treatment indirectly results in an osteoclast-dependent decrease of trabecular bone and increased bone remodeling; and (3) Epo treatment impairs B-cell development through unknown actions of Epo-responsive erythroblast in the BM microenvironment.
Interestingly, the PB erythroid response to Epo was blunted in ZA/Epo-treated mice with decreased RBC numbers (Figure 7E), hemoglobin (Figure 7F), and hematocrit levels (Figure 7G, not significant). ZA/Epo-treated PB was also macrocytic (Figure 7H), which may reflect increased levels of reticulocytes. Taken together, this suggests that bone remodeling is crucial for a normal Epo-induced erythropoietic response to occur. In conclusion, our study reveals a novel role for Epo in coordinating the induction of erythropoiesis and bone remodeling within the BM (Figure 7I).
Discussion
Epo and other erythropoiesis-stimulating agents are widely used clinically. Numerous disease states are also associated with either acute or chronic elevations of endogenous Epo. The consequences of elevated Epo have not been clearly defined in vivo. In this study, we have analyzed the actions of Epo by treating wild-type mice with a relatively low dose of Epo. This led to the observation of a number of previously unrecognized nonerythroid effects associated with elevated Epo, which themselves resulted in significant changes to the BM microenvironment and perturbed hematopoiesis.
The distribution of Epo-R expression is a highly controversial topic and has profound implications for the therapeutic application of Epo.18,44 Recent studies have suggested that the antibodies previously used to detect Epo-R expression are nonspecific.15-18 We have used a functional in vivo lineage-tracing strategy, which detected that Epo-R is first expressed in pre-MegE and pre–CFU-E progenitors and that the erythroid daughters of these cells remain eYFP+ until they enucleate. Our data present in vivo evidence, independent of antibody specificity, for the erythroid-restricted nature of Epo-R expression within hematopoiesis. This is consistent with the most recent reanalysis of Epo-R expression on cell lines.15-18 Importantly, we do not detect in vivo expression of Epo-R on HSCs, myeloid cells, or B-lymphoid populations using the lineage-tracing approach. This is in contrast to a recent report by Shiozawa et al using an antibody based analysis.45 We do detect expression of Epo-R in a small percentage of endothelial cells (∼ 5%), consistent with previously published data.15 In their study, Sinclair et al demonstrate that, although endothelial cells express low levels of Epo-R protein they cannot detect any Epo-induced intracellular signaling in these cells, or any Epo-R on the surface using 125I-rHuEPO binding studies. The detection limit of this assay is however 100 dimers per cell, whereas Um et al have shown that as few as 50 dimers per cell is enough for functional Epo-R signaling.46 The low level of Epo-R induced recombination detected might hence be enough for functional Epo signaling in endothelial cells. Supporting this, Epo treatment resulted in fewer and somewhat enlarged VEGFR3+ vessels in the BM. Notably, transgene-rescued EpoR-null mice expressing Epo-R in the hematopoietic lineages develop normally and are fertile, demonstrating that Epo-Epo-R signaling is not necessary for endothelial cell development.47
Our data provide the most comprehensive analysis to date of the in vivo effects of Epo on erythropoiesis. We showed that erythroid-committed pre–CFU-Es responded to Epo first. This result correlated closely with the in vivo lineage tracing, as this was the earliest population showing robust expression of Epo-R. Although not increased phenotypically, the number of in vitro CFU-Es were increased in both the BM and spleen in response to Epo treatment. There are several factors that could account for this. First, the phenotypically defined population may not encompass all functional CFU-Es. Second, the in vitro assay might not discriminating between the phenotypically defined pre–CFU-E, which is increased, and the CFU-E. The discordance highlights the limitations of using phenotypic markers of erythropoiesis, particularly in the context of alterations in physiology or disease states, and that both phenotypic and functional assays are required. Despite previous observations of Epo effects on platelet production,48 we could not detect any changes in megakaryocytic progenitors in the BM or mature platelets in PB after Epo treatment. We observed a decrease in myeloid cells, which may be accounted for by a mild lineage bias in the myeloerythroid progenitors because of the stressed erythropoiesis induced by Epo. Surprisingly, Epo also altered B-lymphopoiesis, with impairment at the pro B-cell and pre B-cell stage of maturation. Endogenous Epo elevations resulting from acute hemolysis also impacted B-cell maturation, whereas Epo had no effect on B-cell development in vitro, either with or without erythroblasts. As B-cell development depends on osteolineage cells, we considered that the impaired B-lymphopoiesis could be a consequence of the rapid loss of bone. However, blocking bone loss with bisphosphonate treatment failed to rescue B-lymphopoiesis, demonstrating that the B-cell phenotype is independent of the bone remodeling. The mechanism for Epo action on B-lymphoid development remains to be resolved.
The most unexpected and profound nonerythroid phenotype of Epo was on trabecular bone. Within the 10-day treatment, the Epo-treated cohort had a 26% decrease in trabecular bone volume and increased bone remodeling. To the best of our knowledge, this has not been reported previously. In contrast to our results with low doses of Epo, a recent report suggested that Epo treatment resulted in increased bone volume.45 Shiozawa et al45 used very high doses of Epo (4500-6000 U/kg for 28 days, compared with 300 U/kg in the present study), and detected increased bone formation in newborn and very young 4- to 6-week-old animals where bone-modeling rates are high. Importantly, Shiozawa et al45 failed to demonstrate increased hematocrit, the primary physiologic response to Epo, using 10 times the dose we have used. This group also suggested that Epo directly induced signaling in HSCs and osteoclastogenesis. We demonstrate that there is no in vivo expression of Epo-R on HSCs and that Epo is not capable of stimulating in vitro osteoclastogenesis. We further show that PHZ-induced endogenous Epo elevation causes bone remodeling to increase to a similar extent as exogenous Epo, demonstrating that, irrespective of the source, elevations in Epo negatively regulate skeletal homeostasis. The Epo-induced decrease in bone mass observed in this study and the increase in bone mass reported by Shiozawa et al45 are detected at different time points. Although it would be unlikely for the 26% reduction in bone mass that we observed at day 10 to be followed by an increase 18 days later, we assessed bone mass in a second cohort treated at the same low dose for 30 days. Both bone volume and trabecular number were still lower in cohorts of mice treated for 30 days with Epo, as analyzed by histomorphometry (BV/TV: 13.2% ± 0.4% for PBS and 8.6% ± 0.8% for Epo, respectively, 34.5% decrease, P = .0002, hematocrit: 0.51 ± 0.02 L/L for PBS and 0.67 ± 0.02 L/L for Epo, respectively, 32.4% increase, P < .0001, n = 7 and 9 for control and Epo 300 U/kg treated mice, respectively).
It has become increasingly appreciated that the BM microenvironment plays an active and critical role in the regulation of HSCs and hematopoiesis. Within the BM microenvironment, osteolineage cells have attracted particular attention as candidate niche elements.49,50 To our surprise, we could not detect mobilization or any differences in HSCs using both functional and phenotypic assessment, despite the reduction in trabecular bone. This suggests that trabecular bone, at least under certain circumstances, is not necessary for the maintenance of HSCs in the marrow space and that during these periods alternate niches within the BM may be used.
Blockade of bone resorption with ZA blunted the erythroid response to Epo. This unexpected outcome demonstrates that bone remodeling is a crucial physiologic component of the Epo-induced erythropoietic expansion. Collectively, these data reveal a requirement for an increased space in the BM to be coupled to the hematopoietic expansion that occurs in response to a lineage instructive cytokine. Three-dimensional analysis of the BM space has revealed that the erythroid compartment exists in the intertrabecular spaces of the marrow.51,52 This localization may in part explain why the rapid remodeling of the trabecular bone is necessary to allow expanded erythropoiesis in the BM.
The majority of patients receiving Epo have renal disease, and many suffer from renal osteodystrophy. Our results show that Epo is a potent inducer of bone remodeling and that this is necessary for normal erythropoietic response to occur. Changes to bone turnover in the pathologic context of renal osteodystrophy may influence the clinical response to Epo in these patients. In addition, Epo-induced bone loss may have implications for alterations in the BM environment observed in anemic states, such as hemoglobinopathies and diseases associated with elevated Epo, such as refractory anemia of myelodysplastic syndrome. In accordance with our findings, osteoporosis and fractures occur frequently in patients with both sickle cell anemia and β-thalassemia,53,54 although the mechanism for this is unknown.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ankita Gupte, Meryn Chalmers, Ingrid Poulton, Haley Gunn, and Janine Danks for technical assistance; Maria Askmyr for graphical assistance; and Nicole Walsh, Emma Baker, Jörg Heierhorst, Meaghan Wall, and Harshal Nandurkar for valuable discussions.
This work was supported by the Baker Trust (C.R.W.), Leukemia Foundation (C.R.W.), Ian Potter Foundation (major equipment grant) (C.R.W.), and National Health and Medical Research Council Australia (C.R.W.). S.S is supported by the Swedish Research Council and the Swedish Tegger Foundation. C.R.W. was a Special Fellow of the Leukemia & Lymphoma Society, an National Health and Medical Research Council Career Development Award holder, and is the Leukemia Foundation Phillip Desbrow Senior Research Fellow.
Authorship
Contribution: V.G.S. and C.R.W. conceived the study; S.S., L.E.P., T.J.M., V.G.S., and C.R.W. designed experiments; S.S., M.R.R., B.L., T.J., D.J.I., N.A.S., and C.R.W. performed experiments; S.S., M.R.R., T.J., N.A.S., and C.R.W. analyzed data; S.S. and C.R.W. wrote the manuscript; and all authors commented on the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carl R. Walkley, St Vincent's Institute, 9 Princes St, Fitzroy, Victoria 3065 Australia; e-mail: cwalkley@svi.edu.au.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal