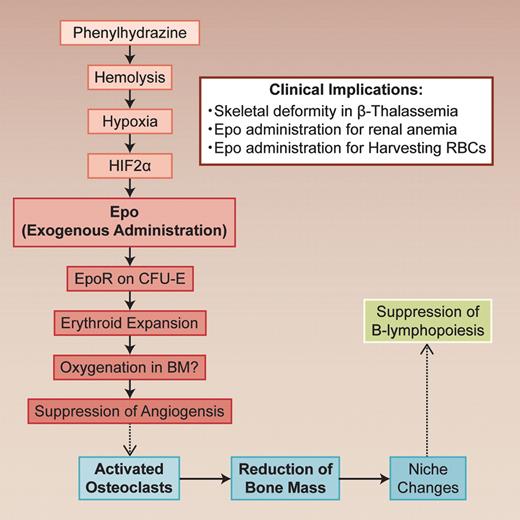
Erythropoietin (Epo) is a polypeptide hormone produced in kidney. It stimulates the proliferation of erythroid progenitor cells in response to hypoxia. In this issue of Blood, the report of Singbrant et al clearly shows the indirect effect of Epo on bone remodeling and its importance for the Epo-induced erythropoietic response.1 The finding adds a new dimension to the regulatory networks controlling eryropoiesis and bone remodeling.
The glycoprotein erythropoietin (Epo) regulates the red blood cell (RBC) mass in response to changes in tissue oxygenation. Epo stimulates erythropoiesis by promoting erythroid precursor cell viability, proliferation, and differentiation, thus enhancing the oxygen-carrying capacity of blood. Singbrant and colleagues clearly show that Epo induces bone remodeling by activating osteoclasts.1 Although the exact mechanisms involved still need to be defined, their conclusion is quite interesting and has a strong impact on the Epo treatment. They treated wild-type mice with a clinically relevant dose (300U/kg) of Epo for 10 days. A significant reduction (26% of control) in trabecular bone volume was observed in Epo-treated mice. When the Epo-induced bone loss was blocked by the inhibition of osteoclast activity, the response of Epo in erythropoiesis was blunted (see figure).
In vivo regulatory nexus in hematopoiesis and bone remodelling. The dotted lines indicate that the mechanisms should be clarified. Professional illustration by Alice Chen.
In vivo regulatory nexus in hematopoiesis and bone remodelling. The dotted lines indicate that the mechanisms should be clarified. Professional illustration by Alice Chen.
Singbrant et al clearly show that Epo receptor (Epo-R) expression was restricted in erythroid lineage cells (erythroid colony forming unit; CFU-E) and endothelial cells by lineage trace experiments in bone marrow. Although osteoclasts and osteoblasts do not express Epo-R, bone remodeling by Epo treatment resulted from increased osteoclast numbers. It is well known that osteoclasts are formed from monocyte/macrophage precursor cells in bone marrow and that RANKL (receptor activator of NF-κB Ligand) and osteoprotegerin (OPG) are essential for osteoclastogenesis.2 However, the levels of these cytokines were not changed with Epo administration. Although the mechanism requires further investigation, Epo does possess an indirect in vivo effect on osteoclasts.
Another interesting finding in this study is the impairment of B-cell development by Epo treatment. This impairment is independent of bone remodeling, because B lymphopoiesis could not be rescued by blocking bone loss with zoledronic acid treatment. Because IL-7 and CXCL12 expression was down-regulated in the bone marrow from mice treated with Epo, there may have been changes in the bone marrow niche. In contrast to myelopoiesis, B lymphopoiesis is easily affected by the changes in the bone marrow niche.
Loss of trabecular bone and B-lymphopoiesis suppression was seen when endogenous Epo elevations in mice were induced by hemolytic anemia caused by phenylhydrazine (PHZ). Induction of Epo gene expression in Epo-producing cells in kidney critically depended on the activation of hypoxia-inducible transcription factors (HIFs), especially HIF-2a.3
This article suggests interplay between erythroid development and skeletal homeostasis. Singbrant and colleagues give a reasonable explanation that bone remodeling during Epo administration is necessary for the acquisition of free bone marrow spaces for erythroid cells expand. This may reflect the phylogenetic process of bone marrow formation within the bone cavity. It is well known that there is a coupling between osteoclasts and osteoblasts. The osteoclast activation/suppression induces osteoblast activation/suppression and vice versa.2 We do not know the exact mechanisms of coupling, but it is hypothesized that endothelial cells intervene this process. Similarly, it is likely that bone remodeling by Epo is mediated by endothelial cells, which express low levels of Epo-R. Indeed, authors found a significant reduction in the number of vessels in the bone marrow. This angiogenic suppression is reasonable, because oxygenation may have been up-regulated after RBCs increased. Such vascular changes may cause a remodeling of the trabecular bone. The mechanism on how osteoclasts are activated is an interesting subject. Another interesting feature provided by this work is the lack of influence of parathyroid hormone (PTH) involvement during Epo treatment. PTH is one of the key regulators for calcium absorption in bone. Four decades ago, Perris et al reported a coupling of the bone marrow response to acute blood loss with the release of PTH.4 However, Singbrant et al showed no significant change of PTH in Epo-treatment.1
Instead, they suggested the involvement of Oncostatin M (OsM), which is produced by erythroblasts.5 OsM is one of the cytokines that bind to and signal through the gp130 co-receptor subunit including IL-6, IL-11, leukemia inhibitory factor (LIF), cardiotrophin-1 (CT-1), and ciliary neutrophic factor (CNTF). Apart from contributing to inflammation, gp130 signaling cytokines also function in the maintenance of bone homeostasis.6
This basic study on the effect of Epo provides us with a new insight into the relationship between hematopoiesis and bone remodeling. The study requires validation of Epo effect on bone in human. However, this potential linkage would explain the skeletal deformity in β thalassemia in which erythropoietin production is enhanced because of hemolysis. In these patients, there is widening of the diploe and lacy trabeculation of the long bones and phalanhges.7 Anemia of renal failure patients is treated with Epo. In these patients, osteodystrophy is a serious problem because of the vitamin D deficiency.2 Thus, although Epo administration is indispensable, we should be careful in treating renal disease patients with Epo, because they show secondary hyperparathiroiism because of the vitamin D deficiency. Treatment with vitamin D, zoledronic acid, and/or Ca-sensing receptor agonists should be considered. During preparation of autologous boold transfusion before surgery, excess administration of Epo should be avoided.
Moreover, it is reported that Epo has a neuroprotective effect.8 It is important to define the Epo-R expression in neuronal cells by antibodies against Epo-R or lineage tracking study. However, as shown by this study, it is very important to analyze the effects of Epo in vivo because other unexpected effects may be uncovered.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal