To the editor:
Human leukocyte antigen (HLA)–DPB1 functions as a classic transplantation antigen.1 In the context of hematopoietic stem cell transplantation (HSCT), when donor T cells recognize host HLA-DP, they can induce a graft-versus-host disease (GVHD) and/or a graft-versus-leukemia (GVL) effect, whereas in the opposite direction, host T cells recognizing the donor can induce rejection (HVG). Accordingly, any possibility to anticipate the nature and strength of an anti-DP T-cell response is crucial in this context.
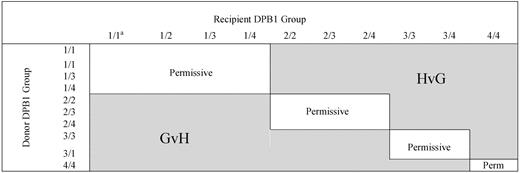
Based on the HLA-DP recognition pattern of several HLA-DPB1*0901-specific T-cell clones, Crocchiolo et al classified HLA-DPB1 alleles according to their predicted “immunogenicity,”2 and using an algorithm deduced from this classification (Figure 1), they showed that the presence of “nonpermissive” HLA-DPB1 mismatches correlated with a significantly increased hazard of acute grade 2-IV GVHD. Unexpectedly, the authors reported that the increased risk of aGVHD was detectable independently of the predicted direction (GVH/GVL or HVG) of the T-cell response. To reconcile the statistical observation with the immunologic hypothesis (the increased risk of GVH when the algorithm predicted the recognition of donor HLA-DP by host T cells), these authors considered the possibility of an indirect pathway for GVH because of cytokine release by host T cells recognizing “immunogenic” HLA-DPB1 on donor antigen-presenting cells (APC).
Algorithm for permissiveness of HLA-DPB1 mismatches in donor-recipients pairs.2 Permissive and nonpermissive HLA-DPB1 disparity according to the algorithm described by Crocchiolo et al. aNumbers indicate the group of the 2 HLA-DPB1 alleles of the donor or the recipient. Group 1: DPB1*09:01,10:01,17:01; group 2: DPB1*03:01, 14:01, 45:01; group 3: DPB1*02:01, 0202, 0203; group 4: others.3 Immunogenicity decreases from group 1 to group 4.
Algorithm for permissiveness of HLA-DPB1 mismatches in donor-recipients pairs.2 Permissive and nonpermissive HLA-DPB1 disparity according to the algorithm described by Crocchiolo et al. aNumbers indicate the group of the 2 HLA-DPB1 alleles of the donor or the recipient. Group 1: DPB1*09:01,10:01,17:01; group 2: DPB1*03:01, 14:01, 45:01; group 3: DPB1*02:01, 0202, 0203; group 4: others.3 Immunogenicity decreases from group 1 to group 4.
In 4 successive studies,3-6 we have in the past assessed the specificities of T-cell clones infiltrating skin biopsies during aGVHD (Table 1). In each situation, T-cell clones specific for host HLA-DP were isolated from the skin at the onset of aGVHD. These studies demonstrated that no mismatch could theoretically be considered as “permissive,” as confirmed in vitro by Rutten et al.7 The finding of up to a 10-fold difference observed between frequencies of T cells directed at a “permissive” versus “nonpermissive” DP mismatch, as argued by Sizzano et al,8 can hardly make a difference in the context of transplantation. T-cell frequency depends on the kinetics of the immune response and is a key physiologic factor in the race against an infection. In the present context of transplantation, the immune target (HLA-DP on host APC) remains present for weeks after the graft, and a 10-fold difference in frequency represents only 3-4 divisions for a T cell, which would take 1-2 days at most.
Nevertheless, a detailed analysis of anti-DP specificities remains particularly interesting and potentially useful (for example, to drive a GVL effect with DP-specific T-cell clones directed against an HLA-DP mismatch in the GVH direction, as we have previously proposed3,4,9 ). In line with HSCT, considering that “alloreactive TCR neither avoid contacting the bound peptide nor focus on the polymorphic residues that are exposed on the outer surface of the allo-MHC a-helices,”10 it would be of great interest to learn more about the set of endogenous peptides presented by HLA-DP alleles in hematopoietic and nonhematopoietic tissues. This may help to improve the targeting of anti-DP allogeneic reaction against the residual disease while avoiding the healthy tissues as much as possible.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Henri Vié, Inserm, 8 Quai Moncousu, 44007 Nantes Cedex 1, France; e-mail: hvie@nantes.inserm.fr.