Abstract
Progression of chronic myelogenous leukemia (CML) to accelerated (AP) and blast phase (BP) is because of secondary molecular events, as well as additional cytogenetic abnormalities. On the basis of the detection of JAK2, CBL, CBLB, TET2, ASXL1, and IDH1/2 mutations in myelodysplastic/myeloproliferative neoplasms, we hypothesized that they may also contribute to progression in CML. We screened these genes for mutations in 54 cases with CML (14 with chronic phase, 14 with AP, 20 with myeloid, and 6 with nonmyeloid BP). We identified 1 CBLB and 2 TET2 mutations in AP, and 1 CBL, 1 CBLB, 4 TET2, 2 ASXL1, and 2 IDH family mutations in myeloid BP. However, none of these mutations were found in chronic phase. No cases with JAK2V617F mutations were found. In 2 cases, TET2 mutations were found concomitant with CBLB mutations. By single nucleotide polymorphism arrays, uniparental disomy on chromosome 5q, 8q, 11p, and 17p was found in AP and BP but not involving 4q24 (TET2) or 11q23 (CBL). Microdeletions on chromosomes 17q11.2 and 21q22.12 involved tumor associated genes NF1 and RUNX1, respectively. Our results indicate that CBL family, TET2, ASXL1, and IDH family mutations and additional cryptic karyotypic abnormalities can occur in advanced phase CML.
Introduction
Loss of heterozygosity (LOH) because of acquired uniparental disomy (UPD) is a commonly observed chromosomal lesion in myelodysplastic/myeloproliferative neoplasms (MDS/MPNs).1,2 Mapping recurrent areas of LOH may aid in the identification of genes harboring mutations, as shown for UPD9p and JAK2V617F mutations.3,4 CBL mutations, often found in a homozygous constellation associated with UPD11q23.3, were most commonly detected in MDS/MPN, including chronic myelomonocytic leukemia (CMML) and juvenile myelomonocytic leukemia.2,5-7 Ring finger domain mutations of CBL family members abrogate their ability to ubiquitinate and inactivate phosphorylated receptor tyrosine kinases.8-11
TET2 mutations are ubiquitous in myeloid malignancies, including MPN, MDS/MPN, MDS, and secondary acute myelogenous leukemia (sAML) and can occur in heterozygous or hemizygous forms, as well as in homozygous forms specifically in the context of UPD4q24.12-16 The TET family of proteins may be involved in the conversion of methylcytosine to hydroxymethylcytosine.17 It is thereby possible that TET proteins regulate the maintenance of methylation-based silencing or prevent aberrant hypermethylation.
Mutations of the polycomb-associated gene ASXL1 were observed in myeloid malignancies, including CMML18,19 and chronic myelogenous leukemia (CML)20 ; unlike TET2 and CBL mutations, mutations in ASXL1 are mostly heterozygous. However, similar to TET2 mutations, ASXL1 mutations may be lead to epigenetic dysregulation. ASXL1 is associated with LSD1, which is involved in histone H3K4 demethylation and thereby chromatin remodeling.21
Somatic mutations of isocitrate dehydrogenases (IDH1 and IDH2), initially described in CNS tumors,22,23 were also found in primary AML24,25 and in sAML evolved from MPN, but not in chronic phase (CP) MPN.26 This distribution pattern suggests a role of IDH1/2 mutations in disease progression.
Although translocations resulting in a BCR/ABL1 fusion gene invariably characterize CML, we stipulated that in analogy to other MDS/MPN entities, JAK2V617F, TET2, ASXL1, CBL, and IDH family mutations may also occur in CML, either contributing to phenotypic heterogeneity within BCR/ABL1-associated chronic myeloid disorders or as secondary events leading to their malignant progression to accelerated phase (AP) or blast phase (BP). Similarly, a higher level of resolution of cytogenetic testing as achieved by single nucleotide polymorphism array (SNP-A)–based karyotyping may show additional chromosomal abnormalities associated with stepwise progression.27,28 Consequently, this study focuses on the combined analysis of additional chromosomal lesions and mutations identified in patients with AP and BP and the association of these defects with clinical features.
Methods
Patients
Informed consent for sample collection was obtained according to protocols approved by institutional review boards of the Cleveland Clinic, Johns Hopkins University, and University of California Los Angeles Medical Center. BM aspirates were collected from 54 patients with 14 CP, 14 AP, and 26 BP (20 myeloid and 6 nonmyeloid; Table 1). Diagnosis was assigned according to classification criteria by the World Health Organization. Because serial samples were not available, only cross-sectional results were reported.
Metaphase cytogenetics
Cytogenetic analysis was performed on marrow aspirates or peripheral blood or both according to standard methods; 20 metaphase spreads were examined per patient. Chromosome preparations were G-banded with the use of trypsin and Giemsa, and karyotypes were described according to the International System for Cytogenetic Nomenclature.29
SNP-A analysis
Affymetrix Genome-Wide Human SNP Array 6.0 and Illumina HumanCytoSNP-12 were used for SNP-A analysis of BM DNA as previously described.30 Sufficient DNA was available from 26 of 40 patients with AP (N = 12) and BP (N = 14). Briefly, signal intensity was analyzed, and SNP calls were determined with GeneChip Genotyping Analysis Software Version 4.0 (GTYPE). Genotyping console v3.0 (Affymetrix) and KaryoStudio (Illumina) were used for analysis of 6.0 arrays and HumanCytoSNP-12, respectively. Germline-encoded copy number variations and nonclonal areas of UPD were excluded from further analysis with the use of a bioanalytic algorithm, based on lesions identified by SNP-A13,31 in an internal control series (N = 1003) and reported in the Database of Genomic Variants (http://projects.tcag.ca/variation/). Size and location criteria (telomeric > 8.7 Mb and interstitial ≥ 25 Mb in size) were used for identification of somatic UPD as previously described.7 The reference genome used for annotation was NCBI36/hg18 (March 2006).32
JAK2, CBL family, TET2, ASXL1, and IDH family mutational screening
We checked mutational status of JAK2, CBL family, TET2, ASXL1, and IDH family genes in all 54 enrolled patients (Table 1). Screening for the JAK2V617F mutation was performed with a DNA tetraprimer amplification refractory mutation system assay as previously described.2,33 For CBL (exons 8-9), CBLB (exons 9-10), TET2 (all coding exons), ASXL1 (exon 12), IDH2 (exon 2), and IDH2 (exon 4), direct genomic sequencing was performed as previously described.7,13,26,34 Bidirectional sequencing was performed by standard techniques with the use of an ABI 3730xl DNA analyzer (Applied Biosystems). All mutations were scored as pathogenic on the basis of the observation that they were not detected in normal samples and were not found in a publically available SNP database,35 or they were not reported as SNPs in previous publications.
Statistical analysis
For comparison of the clinical features between disease groups, categorical and continuous variables were analyzed with the Fisher exact test and Mann-Whitney U test, respectively. Overall survival was analyzed with Kaplan-Meier statistics and compared by log-rank test and generalized Wilcoxon test.
Results
CBL family, ASXL1, TET2, and IDH family mutations in CML
When we performed mutational screening for JAK2, CBL family, TET2, ASXL1, and IDH family genes in CML, we identified mutations in a number of AP CML. One CBLB and 2 TET2 mutations in AP (N = 14), and 1 CBL, 1 CBLB, 4 TET2, 2 ASXL1, and 2 IDH family mutations in myeloid BP (N = 20). In contrast, when patients with CP (N = 14) were screened, no mutations were found (Tables 1–2). JAK2V617F mutation was not present in any case. Interestingly, TET2 mutations were concomitantly present in 2 cases with CBLB mutations. Similarly, 1 myeloid BP case was characterized by an ASXL1 nonsense mutation as well as an IDH1 mutation (Table 2). An overview of the mutations found in the affected genes is shown in Figure 1. Mutant cases and corresponding cytogenetic features are presented in Table 2.
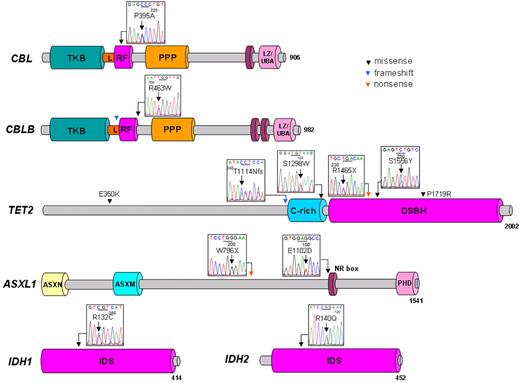
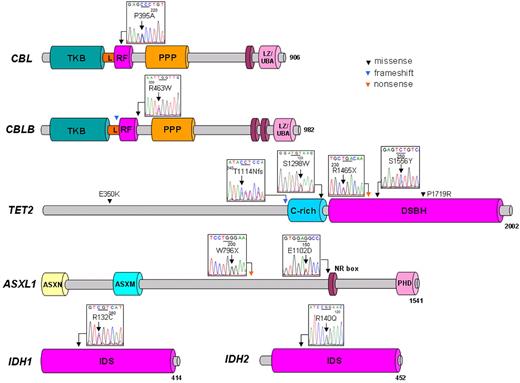
Distribution of mutations in tested genes in 10 patients with CML. Schematic representation shows the main domains, primarily the tyrosine kinase binding (TKB) domain, linker sequence (L), RING finger domain (RF), proline-rich region (PPP), and leucine zipper (LZ)/ubiquitin-associated domain (UBA) of the CBL family, cysteine (C)–rich domain, and double-stranded β helix (DSBH) domain of TET2, additional sex comb (ASX) N-terminal (ASXN) domain, ASX-middle (ASXM) domain, nuclear receptor coregulator binding (NR box) motif, plant homeo domain (PHD) of ASXL1, and isocitrate dehydrogenase superfamily (IDS) region of IDH1/2. Genomic sequencing of protein-coding regions and splice sites showed missense (black), nonsense (orange), and frameshift mutations (blue) in CBL, CBLB, TET2, ASXL1, IDH1, and IDH2 genes. All base pair changes identified occurred in a heterozygous constellation.
Distribution of mutations in tested genes in 10 patients with CML. Schematic representation shows the main domains, primarily the tyrosine kinase binding (TKB) domain, linker sequence (L), RING finger domain (RF), proline-rich region (PPP), and leucine zipper (LZ)/ubiquitin-associated domain (UBA) of the CBL family, cysteine (C)–rich domain, and double-stranded β helix (DSBH) domain of TET2, additional sex comb (ASX) N-terminal (ASXN) domain, ASX-middle (ASXM) domain, nuclear receptor coregulator binding (NR box) motif, plant homeo domain (PHD) of ASXL1, and isocitrate dehydrogenase superfamily (IDS) region of IDH1/2. Genomic sequencing of protein-coding regions and splice sites showed missense (black), nonsense (orange), and frameshift mutations (blue) in CBL, CBLB, TET2, ASXL1, IDH1, and IDH2 genes. All base pair changes identified occurred in a heterozygous constellation.
SNP-A–based detection of accessory karyotypic abnormalities in AP and BP
In addition to mutations, progression of CML may also be associated with the acquisition of additional, previously cryptic, chromosomal abnormalities. SNP-A allows for the identification of not only submicroscopic copy number changes but also UPD, not amenable to detection with the use of routine metaphase cytogenetics. FISH or reverse transcription PCR confirmed the presence of a BCR/ABL1 fusion gene in all of these patients. We focused on additional karyotypic abnormalities other than t(9;22) in AP and BP. With the use of metaphase cytogenetics, additional chromosomal aberrations were found in 21 of 40 patients (53%). Additional unbalanced (deletion and addition) defects, balanced (translocation and inversion) defects, and both were found in 28%, 15%, and 10% of cases, respectively (Figure 2). The most common recurrent defects included abnormalities on chromosomes 3 (15%) and 22 (15%), as well as common lesions on chromosomes 7 (10%), 8 (8%), 9 (8%), 11 (8%), and 18 (8%).
Chromosomal regions affected in patients with AP and BP. The left pie diagram shows proportion of patients with chromosome aberrations detected by standard metaphase cytogenetics (MC) [only Philadelphia chromosome (Ph; white), unbalanced (black), balanced (light gray), and both classes of abnormalities (dark gray)] in persons with AP and BP (N = 40), whereas the right pie chart represents the abnormalities by SNP-A karyotyping [normal (white) or abnormal (black); N = 26].
Chromosomal regions affected in patients with AP and BP. The left pie diagram shows proportion of patients with chromosome aberrations detected by standard metaphase cytogenetics (MC) [only Philadelphia chromosome (Ph; white), unbalanced (black), balanced (light gray), and both classes of abnormalities (dark gray)] in persons with AP and BP (N = 40), whereas the right pie chart represents the abnormalities by SNP-A karyotyping [normal (white) or abnormal (black); N = 26].
SNP-A–based karyotyping was available on patients with AP (N = 12) and BP (N = 14). For the purpose of this study, we only included lesions that did not overlap with either copy number variations or germline-encoded regions of homozygosity detected in an internal control cohort or external databases (see “SNP-A analysis”); 21 gains, 18 losses, and 4 regions of UPD were identified in the patients (Tables 3,Table 4–5). In general, SNP-A confirmed the results of metaphase cytogenetics with regard to known unbalanced defects. Additional copy number abnormalities, including microdeletions, were also found in 58% of all examined cases (67% and 50% in AP and BP, respectively) by SNP-A.
Recurrent lesions were detected on chromosomes 1, 8, 9, 17, and 22 by SNP-A. However, these lesions were overlapping those detected by metaphase cytogenetics. Microdeletions on chromosomes 17q11.2 (3.84 Mb) and 21q22.12 (1.21 Mb), which were undetectable by metaphase cytogenetics, involved tumor-associated genes NF1 and RUNX1, respectively (Figure 3; Table 4). In 1 patient with AP, a microdeletion of 11q23.3 was present, but no mutation in CBL, mapping to this interval, was detected. Deletions flanking the ABL1 and BCR genes (chromosome 9 and 22) were observed in 2 cases with der(22)t(9;22) or der(9)t(9;22) by metaphase cytogenetics and were previously described by others.36,37 Gains, including whole chromosome 8 and 17q24.3 (0.76 Mb), were found in 3 cases (Table 3). Regions of UPD included UPD5q, 8q, 11p, and 17q, but no UPD involving 11q (CBL) and 4q (TET2) regions were found confirming the heterozygous nature of the corresponding mutations (Figure 3; Table 5). Of note is that none of the cases showed the presence of somatic UPD9p (associated with homozygous JAK2V617F mutation).
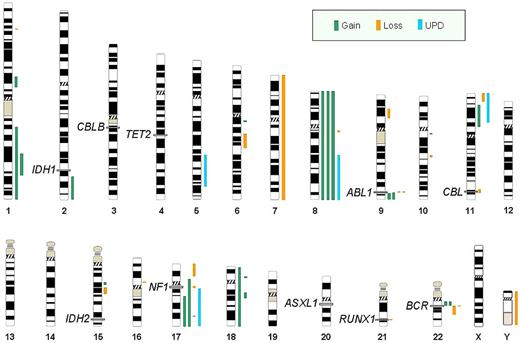
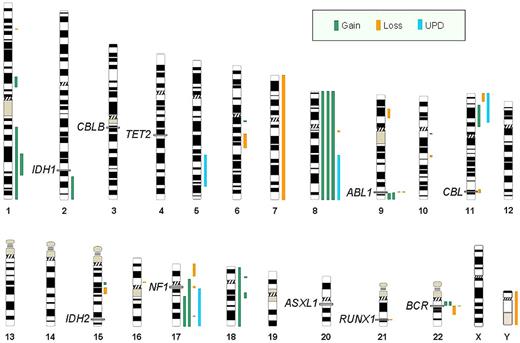
Overview of all genetic aberrations found by SNP-A karyotyping in 26 patients with AP and BP. Each line represents chromosomal aberration: green indicates copy number gains; orange, losses; and blue, regions of UPD. Exact location of IDH1, CBLB, TET2, ABL1, CBL, IDH2, NF1, ASXL1, RUNX1, and BCR are noted on chromosomes 2q, 3q, 4q, 9q, 11q, 15q, 17q, 20q, 21q, and 22q, respectively.
Overview of all genetic aberrations found by SNP-A karyotyping in 26 patients with AP and BP. Each line represents chromosomal aberration: green indicates copy number gains; orange, losses; and blue, regions of UPD. Exact location of IDH1, CBLB, TET2, ABL1, CBL, IDH2, NF1, ASXL1, RUNX1, and BCR are noted on chromosomes 2q, 3q, 4q, 9q, 11q, 15q, 17q, 20q, 21q, and 22q, respectively.
Clinical characteristics in patients with CBL family, TET2, ASXL1, and IDH family mutations
Newly detected molecular lesions associated with AP and BP may change the biology and thereby clinical features of affected cases. Mutant and wild-type (WT) cases did not differ by age or duration of the disease. Although karyotypic abnormalities were detected by SNP-A in all cases with TET2 mutations, there was no significant difference in the frequencies between mutant and WT groups (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Clinically, there was no significant difference in the treatment regimen administered to patients with and without specific mutations. All patients with CBL family and 4 of 5 patients with TET2 mutations were resistant to imatinib mesylate. However, dasatinib was effective in a patient with a mutation of TET2. Overall survival of patients with mutations did not differ from patients without mutations (median survival of progressed CML: 49, 36, 46, 67, and 41 months in patients with CBL family, TET2, ASXL1, IDH family, or no mutations, respectively; supplemental Table 1). Of note is that BCR-ABL1 kinase domain mutations were detected in 9 of 10 patients with imatinib mesylate resistance. In these 9 cases, 3 TET2 and 2 CBLB mutations were detected. In 1 patient resistant to imatinib mesylate without BCR-ABL1 kinase domain mutation, CBL mutation was present (Table 2).
Discussion
Mutations in JAK2, CBL, CBLB, TET2, ASXL1, IDH1, and IDH2 have been found in various myeloid malignancies, including MDS/MPN, MPN, and sAML. This is the comprehensive mutational analysis of these genes in CML, based on the theory that these mutations may contribute to the progression of CML to AP and BP. Among the genes studied, TET2 was the most frequently mutated, and the mutations were distributed in AP and BP.
CBL family mutations found in AP and BP were located in or next to the ring finger domain or linker sequence, similar to those observed in MDS/MPN. These regions are completely conserved in CBL orthologs as well as in 2 other human CBL family members. Of 3 CBL family mutations, 2 were missense substitutions and 1 was a frameshift variation with deletion of 144 bp in intron 8 and exon 9. Previously, in CMML and related disorders we identified UPD11q and associated homozygous CBL mutations.2,6,38 However, we also noted that heterozygous mutations or alterations of other closely related E3 ubiquitin ligases, such as CBLB and CBLC, may be found in some patients with otherwise indistinguishable morphologic features.7 Moreover, heterozygous CBL mutations are also described in juvenile myelomonocytic leukemia.5,39,40 In this report, CBL family mutations were present only in aggressive forms of CML. In contrast, they were found in both CMML and CMML-derived sAML,7 suggesting that heterozygous CBL family mutations may play an initiating role in MDS/MPN but are accessory in the pathogenesis of evolution into aggressive phase of CML.
In all patients with TET2 mutations, additional chromosomal lesions were found by SNP-A. Of the 6 TET2 mutations identified, 4 (67%) were missense substitutions, 1 (17%) was frameshift, and 1 (17%) produced a stop codon and were located within the N-terminus as well as in a conserved DSBH 2OG-Fe(II)–dependent dioxygenase domain. The presence of nonsense and frameshift mutations suggests that these changes result in inactivation, consistent with putative tumor suppressor functions, whereas heterozygous mutations indicate that the WT allele is not completely protective. In other myeloid malignancies, the distribution pattern and types of mutations are similar,13,15,16 but they occur in both homozygous and heterozygous configurations. Because no TET2 mutations were identified in CP, these mutations might represent an additional pathogenic event. Recently, TET2 mutations were shown to be a late event in MDS, MDS/MPN, and MPN, because they are rarely present in low-grade MDS and frequent in secondary AML13,41,42 in contrast to the initial findings that mutations in TET2 may precede acquisition of a JAK2 mutation.12 Consequently, the role of TET2 mutations as initial or cooperating events is not settled and may differ in various myeloid malignancies.
ASXL1 mutations are reported in 11% of MDS, 43% of CMML, and 47% of secondary AML.18,43 Recently, ASXL1 mutations were seen in both BP as well as CP,20 whereas in our series these mutations were detected in only AP and BP. Combining our findings with the previously reported results, ASXL1 mutations were seen in 4 of 48 CP samples and 5 of 47 BP cases. Consequently, it is still uncertain whether ASXL1 mutations constitute an early or a secondary event. Because knockout mice did not develop myeloid malignancies,44 ASXL1 mutations might cooperate with other defects such as the BCR-ABL1 fusion gene or other mutations.
IDH family mutations confer an enzymatic gain of function that increases 2-hydroxyglutarate; consequently, heterozygous acquisition of these mutations may be sufficient to facilitate malignant progression.45,46 We have detected canonical IDH mutations [R132 (IDH1) and R140 (IDH2)] in aggressive stages of CML analogous to gliomas and AML.23,26 This suggests their secondary role in acquisition of a more malignant phenotype.
In isolated case reports, JAK2V617F mutations were found in patients with CML.47 In some instances, the JAK2V617F-positive clone evolved when BCR-ABL1 allelic burden decreased after treatment for CML.48,49 However, larger studies suggest that JAK2V617F mutations do not occur in CML.50 In agreement with this study, neither this mutation nor UPD9p was found in our cohort of patients; thus, it may be safe to conclude that JAK2V617F mutations are less common in CML than other genes examined in this study.
In 3 cases we observed a combination of mutations in 2 genes. The coexistence of CBLB and TET2 mutations in 2 cases suggests that these might be mutually supportive of each other in the AP phase of CML or that sequential clonal events lead to stepwise progression and more aggressive disease. We also found a combination of IDH1 and ASXL1 mutations in 1 of our patients with BP, suggesting that both mutations contribute to the clonal advantage.
Most of the CBL, TET2, and ASXL1 mutations were unique. Similarly, frameshift and nonsense mutations are unlikely to be germline variants. Theoretically, it is possible that some of the missense alterations found here represent novel germline polymorphisms. However, they were not found in any SNP databases and also not encountered in any of our previous sequencing studies (> 400 patients sequenced to date) or described by others, suggesting that their frequency would be exceedingly low.
Additional nonrandom chromosomal abnormalities (eg, affecting chromosome 8, 17, 19, and 22) are often observed during progression of CML to aggressive phase.51 To date, 3 different groups reported SNP-A–based surveys of chromosomal lesions in CML. One analyzed 45 tyrosine kinase inhibitor-resistant CML cases and showed that several acquired regions of UPD and recurrent deletions, as well as duplication of the BCR-ABL1 gene and trisomy 8, previously found by metaphase cytogenetics.52 Although in our study SNP-A results were available in a representative subset of patients, the findings are in agreement with previous studies.20,52 We demonstrate that some defects are recurrent, for example, microdeletions in 9q and 22q and a recurrent copy number gain on 17q. Most importantly, we showed regions of LOH associated with oncogenes and proto-oncogenes, such as deletion of 17p13.3p11.2, containing the TP53 locus, as previously reported in myeloid BP.53 In 2 cases with lymphoid phenotype studied by SNP-A, we were unable to find previously described homozygous deletion of IKZF1,54 but a heterozygous deletion 9p21.3p13.2 in CDKN2A region was present in a patient with myeloid BP. However, no cryptic recurrent lesions were identified by SNP-A, and in general shared defects were detectable by both metaphase cytogenetics and SNP-A karyotyping
Although there was a general concordance between SNP-A and metaphase cytogenetics in most examined cases, some differences were found. Discordance is probably related to the differences in the sensitivity of these methods as previously described.27,30 For example, in patient with AP (no. 30), del(5)(q22q23) in 19 metaphases was not detected by SNP-A. We conceived this study fully aware that the SNP-A technology is not designed to replace metaphase cytogenetics.
When clinical features were studied, there was no significant difference in overall survival between patients with mutations and WT. This may be because of the generally poor prognosis of patients with CML with advanced malignancy refractory to imatinib mesylate. A screen for BCR-ABL1 tyrosine kinase mutations helped to identify a patient resistant to imatinib mesylate with a mutation of CBL who had a WT BCR-ABL1 kinase domain, suggesting that mutations in other genes might contribute to refractoriness to tyrosine kinase inhibitors. However, it is probable that imatinib mesylate resistance and progression to AP or BP may be controlled by separate molecular events.
It is possible that, because of limited sensitivity of Sanger sequencing, mutations in these genes are present in a much higher proportion of patients. For example, although we detected CBL mutations in 13%-15% of CMML cases with the use of routine sequencing, application of next-generation sequencing resulted in a higher detection rate of 25%.55 Clearly, our report does not settle this issue, and the mutation status of genes investigated in our study should be prospectively and serially evaluated in a larger cohort of patients with the use of deep sequencing approaches.
In conclusion, CBL family, TET2, ASXL1, and IDH family mutations as well as additional unbalanced chromosomal abnormalities not seen by metaphase cytogenetics can occur in advanced phase CML with myeloid phenotype. These mutations probably represent secondary lesions that contribute to aggressive features in AP and BP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (grant RO1HL-082983; J.P.M.), (grant U54 RR019391; J.P.M.), and (grant K24 HL-077522: (J.P.M.); Department of Defense (grant DOD MPD510343 (M.A.M.); and a grant from the AA & MDS International Foundation and the Robert Duggan Charitable Fund (J.P.M.).
National Institutes of Health
Authorship
Contribution: H.M., A.M.J., and J.P.M. designed and performed research, analyzed data, and wrote the paper; M.A.M., A.A., and R.P. designed research and wrote the paper; C.O., S.D., H.C., C.P., J.N., H. Szpurka, and E.H. performed research; and B.P., H. Siddaiah, and M.S. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaroslaw P. Maciejewski, Taussig Cancer Institution/R40, 9500 Euclid Ave, Cleveland OH 44195; e-mail: maciejj@ccf.org.

![Figure 2. Chromosomal regions affected in patients with AP and BP. The left pie diagram shows proportion of patients with chromosome aberrations detected by standard metaphase cytogenetics (MC) [only Philadelphia chromosome (Ph; white), unbalanced (black), balanced (light gray), and both classes of abnormalities (dark gray)] in persons with AP and BP (N = 40), whereas the right pie chart represents the abnormalities by SNP-A karyotyping [normal (white) or abnormal (black); N = 26].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/21/10.1182_blood-2010-06-292433/4/m_zh89991169570002.jpeg?Expires=1769294677&Signature=14aG1tEal0BUMR~gMSOrWIET0td~y7IbUGLPAZ73MGfGQI7XUw1x1~UG2rT-4yAIXrmtUF4s97o-uWLAOcVm05LiwCUIwMxUwkD~LFaXoSgqf0C4RuQ65jnncnx9TQw0KFPJNLWz1fLOSCLFdEk869zB5ipxDgUyEGB33gtLqoBQiyCb1HgKh1TyOnTN~soFH8ehbFkI1atnJ0FwvUPpgiRH7r8G~hEumqJLezT6APSDIOMxYR94AyBbeTRSyF7nbJk3MKrJoM~OonQlsg~WvKSLrUO8sIDGONIQ9Hdv8gkf~fjbBn5SeFEXXnLWyAgsSvELfAFgan9lR7JsO05hIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Chromosomal regions affected in patients with AP and BP. The left pie diagram shows proportion of patients with chromosome aberrations detected by standard metaphase cytogenetics (MC) [only Philadelphia chromosome (Ph; white), unbalanced (black), balanced (light gray), and both classes of abnormalities (dark gray)] in persons with AP and BP (N = 40), whereas the right pie chart represents the abnormalities by SNP-A karyotyping [normal (white) or abnormal (black); N = 26].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/21/10.1182_blood-2010-06-292433/4/m_zh89991169570002.jpeg?Expires=1769294678&Signature=nUy0EA4tnfgehN~~oclUHx6w6i3aPnRUmnv3d75Zo37sLC2vNiLRbL~NRRTzGOAiYSa1r4yo8X8q9-YWK~OIOg6yhgzXFkJQw11OVR-Mg6WvE2ORCJ76LfZVCEFoFSM0KfFVdl6NlDG1umHp8tuZ9F6UEjlH2jE2k3pXrCe8LzYln4QL~2wpHVmxt~V7Aoq0BeqK6QEFoSms0eqjHxvMYkk8ySrCkRWpibnL05Kb7OF6iifVC82ynBOnN6M85WcCDW1oHas4MB~0pBOM6FadWFJBYa3x9HI15qwSZRqnMRy5RHdqoKT0hPtyvmMoNCsZ-tc-kUZuyrt2d-zSCl5f3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)