To the editor:
In the July 29, 2010, issue of Blood, Pillay et al presented a novel study on the kinetics of neutrophils in humans.1 Their study used deuterium in heavy water to label proliferating cells in the marrow. Using a first-order model, the authors found that the neutrophil postmitotic marrow transit time was about 5.8 days, consistent with many other reports.2,3
Pillay and coworkers reported, however, that the normal blood neutrophil half-life is 3.75 days.1 This estimate of the blood neutrophil half-life is approximately 10 times longer than that previously shown using several different methodologies.2-7 The authors suggested that this discrepancy could indicate alteration of cells because of manipulation during exogenous labeling or abnormal homing conditions in the previous studies.
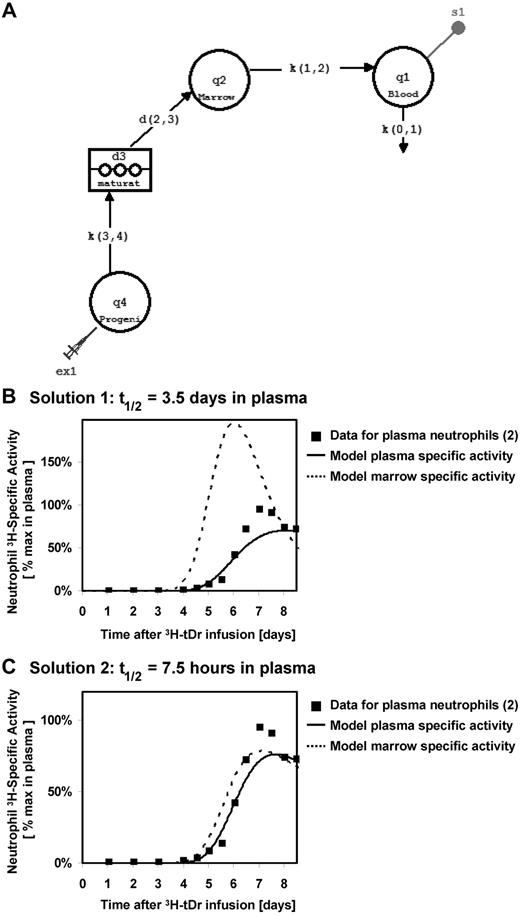
We propose that there is an alternate interpretation of the data of Pillay et al. The bone marrow is a delay compartment that was not measured experimentally by Pillay et al. Using a multicompartment model, with the pool of marrow neutrophils serving as the precursor input for a blood plasma pool, we performed simulations using data from Dancey et al.2 In this model, progenitor cells incorporate label as they proliferate, and then mature in the bone marrow before being released into the blood pool, where the labeled granulocytes are sampled experimentally (Figure 1A).
Tracking neutrophils from bone marrow to blood, modeling using built-in components in SAAM II. (A) Model describing the flux of labeled neutrophils through the hematopoiesis system, using built-in components in SAAM II. Distribution of labeled neutrophils between marrow and blood assuming blood half-lives of (B) 3.5 days and (C) 7.5 hours. In both cases, the model fits the specific activity in the plasma neutrophils to published specific activity measurements for thymidine-tagged neutrophils in circulation.2
Tracking neutrophils from bone marrow to blood, modeling using built-in components in SAAM II. (A) Model describing the flux of labeled neutrophils through the hematopoiesis system, using built-in components in SAAM II. Distribution of labeled neutrophils between marrow and blood assuming blood half-lives of (B) 3.5 days and (C) 7.5 hours. In both cases, the model fits the specific activity in the plasma neutrophils to published specific activity measurements for thymidine-tagged neutrophils in circulation.2
We found that the model can be solved with many sets of rate constants that produce similar model fits to the specific activity of granulocytes measured in the blood. There are 2 solutions that are particularly notable. In solution 1, the blood pool is the rate-limiting kinetic step, and the result is a blood half-life of 3.5 days, consistent with the results presented by Pillay et al (Figure 1B). Note that the specific activity in the marrow rises earlier and decays faster than that in the blood because the latter step is rate-limiting. For solution 2, if one imposes a blood neutrophil half-life time of 7.5 hours, consistent with literature values (Figure 1C), proliferation and differentiation of neutrophils through the marrow become the rate-limiting kinetic step. In this situation, kinetics in the delay compartment are responsible for both the lag in the first appearance of labeled neutrophils in blood and the shape of the blood specific activity curve. Moreover, the specific activity in the bone marrow is nearly equilibrated with that in the blood under this solution, because the former is rate-limiting.
The authors' published data in mice show that the 2H-enrichment of mature neutrophils in the marrow matches well with that in the blood, suggesting that the measured appearance of labeled cells in the blood reflects their kinetics in the marrow. These results are consistent with flux of labeled populations through the marrow being the dominant kinetic step in the system. Thus, we believe that deuterium measurement of neutrophil kinetics yields results that are, in fact, quite harmonious with previous reports and likely reflect primarily kinetics of cellular flux through the bone marrow compartment.
Authorship
Conflict-of-interest disclosure: K.W.L., S.M.T., and C.L.E. are employees of KineMed Inc. M.K.H. owns stock in, is on the Scientific Advisory Board of, holds patents from, and receives consulting income from KineMed Inc. D.C.D. conducts sponsored research for Merck & Co Inc as approved by the University of Washington.
Correspondence: David C. Dale, MD, Department of Medicine, University of Washington, Box 356422, 1959 NE Pacific St, Rm AA-522, Seattle, WA 98195; e-mail: dcdale@u.washington.edu.