Abstract
Previous investigations of cancer survivors report that the cumulative incidence of subsequent leukemia plateaus between 10 and 15 years after primary therapy. Risk beyond 15 years has not been comprehensively assessed, primarily because of lack of long-term follow-up. Among 5-year survivors from the Childhood Cancer Survivor Study cohort, 13 pathologically confirmed cases of subsequent leukemia occurred ≥ 15 years after primary malignancy, with a mean latency of 21.6 years (range, 15-32 years). Seven were acute myeloid leukemia (2 acute promyelocytic leukemia with t(15;17), 2 with confirmed preceding myelodysplastic syndrome), 4 acute lymphoblastic leukemia (2 pre-B lineage, 1 T cell, 1 unknown), and 2 other. Two acute myeloid leukemia cases had the 7q− deletion. The standardized incidence ratio was 3.5 (95% confidence interval, 1.9-6.0). Median survival from diagnosis of subsequent leukemia was 2 years. This is the first description of a statistically significant increased risk of subsequent leukemia ≥ 15 years from primary diagnosis of childhood cancer.
Introduction
Almost 80% of children diagnosed with cancer will achieve 5-year survival, with the majority becoming long-term survivors.1 These survivors have an increased risk of subsequent malignant neoplasms.2,3 Reports evaluating cancer survivors have found that the cumulative incidence of subsequent leukemia, predominantly acute myeloid leukemia (AML), plateaus at approximately 2% 10 to 15 years after primary cancer therapy.4 Treatment-related AML is associated with exposure to alkylating agents, typically preceded by myelodysplastic syndrome and a loss or partial deletion of tumor suppressor genes on chromosomes 5 or 75,6 and epipodophyllotoxins, which are associated with translocations of the MLL gene at chromosome band 11q23.7-9 Anthracyclines have also been linked to leukemia with 11q23 abnormalities when used in conjunction with alkylator therapy.10 Time to development of alkylating agent-induced leukemia is 5 to 7 years from primary cancer, whereas epipodophyllotoxin-associated leukemia has a latency of 2 to 3 years.11,12
Risk of subsequent leukemia ≥ 15 years beyond initial cancer diagnosis has not been comprehensively assessed, in part because of the lack of sufficient sample size and extended surveillance. The Childhood Cancer Survivor Study (CCSS) cohort offers a unique opportunity to evaluate a large population of 5-year survivors with a variety of primary malignancies and follow-up into adulthood. We report the first description of a statistically significantly increased risk of subsequent leukemia occurring ≥ 15 years from treatment of a primary malignancy.
Methods
The CCSS is a retrospective cohort study, with longitudinal follow-up of 14 358 5-year survivors of childhood cancer treated at 26 institutions in the United States and Canada between 1970 and 1986. CCSS methodology was previously described.13,14 The CCSS was approved by the institutional review boards of all participating institutions. Subsequent leukemia includes leukemias occurring ≥ 5 years from diagnosis, initially ascertained through self- or proxy-report questionnaires, and confirmed by pathology report, death certificate, or other medical records. Relapses of primary leukemia, based on comparison of pathologic reports, were considered recurrences, not subsequent leukemia. Bone marrow samples and cyotgentic reports were acquired for 10 of the 13 cases of leukemia occurring ≥ 15 years from diagnosis of primary malignancy. Bone marrow samples were centrally reviewed by the CCSS pathologist (S.H.) to further validate the diagnoses. Consent for release of initial cancer treatment records was obtained from 10 of the 13 cases. Cumulative incidence estimates, based on patients at risk at a given time point, were calculated from 5 years after childhood cancer diagnosis to first occurrence of leukemia, treating death as a competing risk. The standardized incidence ratio (SIR) and absolute excess risk were derived using age, sex, and calendar year specific rates from the Surveillance Epidemiology and End Results database.1
Results and discussion
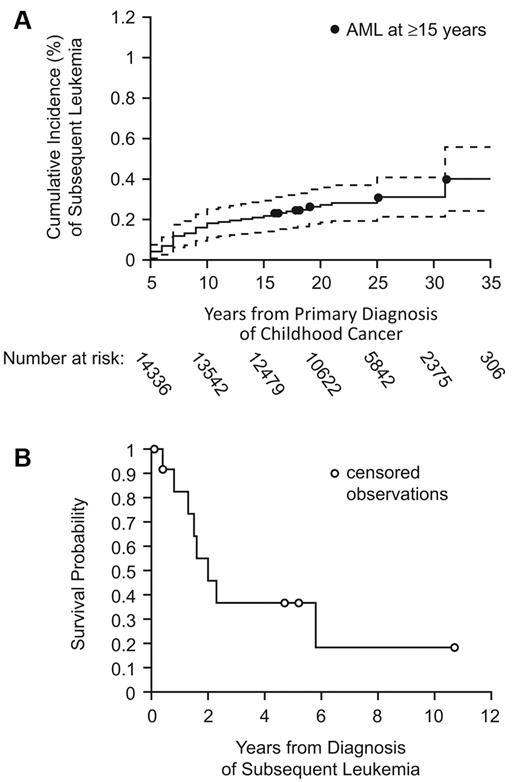
Of the 14 358 survivors in the CCSS, 43 developed subsequent leukemia ≥ 5 years from primary diagnosis; 25 occurred 5 to 10 years, 5 at 10 to 15 years, and 13 at ≥ 15 years. The 30-year cumulative incidence for development of subsequent leukemia was 0.31% (95% confidence interval [CI], 0.21%-0.41%; Figure 1A). Compared with the general population, CCSS survivors had a greater than 6-fold increased risk (SIR = 6.3; 95% CI, 4.6-8.5) for developing leukemia. Risk was highest between 5 and 10 years (SIR = 15.4; 95% CI, 10.0-22.8) and remained significantly higher than the background incidence ≥ 15 years from primary diagnosis (SIR = 3.5; 95% CI, 1.9-6.0). The absolute excess risk of leukemia as a subsequent malignant neoplasm ≥ 15 years in CCSS survivors was 0.02 cases per 1000 person-years. Risk of AML ≥ 15 years was increased (SIR = 5.3; 95% CI, 2.1-10.9).
Long-term incidence and overall survival of subsequent leukemia. (A) Cumulative incidence with 95% CIs of subsequent leukemia among 5-year childhood cancer survivors in the CCSS cohort. (B) Overall survival after diagnosis of subsequent leukemia ≥ 15 years from diagnosis of childhood cancer.
Long-term incidence and overall survival of subsequent leukemia. (A) Cumulative incidence with 95% CIs of subsequent leukemia among 5-year childhood cancer survivors in the CCSS cohort. (B) Overall survival after diagnosis of subsequent leukemia ≥ 15 years from diagnosis of childhood cancer.
Among the 13 subsequent leukemia cases occurring ≥ 15 years (Table), mean age at diagnosis was 31.2 years (range, 18-51 years) with mean latency of 21.6 years (range, 15-32 years). Subsequent leukemias included 7 cases of AML (2 with documented preceding myelodysplastic syndrome and 2 acute promyelocytic leukemia, both with documented t(15;17) translocation), and 4 acute lymphoblastic leukemia (2 pre-B lineage, one T cell, and one unknown). For the remaining 2 cases, one was T-cell large granular leukemia, and one was only verified as “leukemia” via death certificate, without supporting documentation or pathology slides. Two patients with subsequent AML had the 7q− deletion. Among cases of subsequent acute lymphoblastic leukemia, one had a complex karyotype that included t(9;22) and another had a p53 mutation. Sarcomas (n = 5) and Hodgkin lymphoma (n = 4) were the most common primary diagnoses, with only one patient having had an initial diagnosis of leukemia. Six patients with subsequent leukemia received radiation therapy, which was the sole therapy in 2 patients. Six patients received both an alkylating agent and anthracycline, and none received epipodophyllotoxins. Median survival time after subsequent leukemia diagnosis was 2 years (range, 0.4-5.8 years, Figure 1B).
In this study of aging adult survivors of childhood cancer, we identified a statistically significant increased risk of subsequent leukemia ≥ 15 years from primary cancer therapy. This is contrary to numerous reports in the literature on treatment-related leukemia, which suggest that the cumulative incidence plateaus after 10 years.4,7,15-22 Similar to patients with subsequent leukemia occurring in the first 10 years after diagnosis, those ≥ 15 years have a poor prognosis with median survival of 2 years. Median survival of therapy-related AML is 5 to 11 months.12
It is unclear why this long latency exists. Studies in atomic bomb exposed children show a peak incidence of leukemia at 5 to 7 years after exposure, and the incidence decreases thereafter, returning to the population risk at 15 years.23 As radiation therapy was the most common exposure for these late-occurring subsequent leukemias, one hypothesis is that these cases undergo a series of alterations in oncogenes or tumor suppressor genes, which may require an extended time period and additional environmental exposures to create a prolific clone. In addition, it is possible that this group of patients may have an underlying genetic predisposition that was either not tested for or is not yet known. Polymorphisms in the NQO1 gene are associated with treatment-related AML (t-AML) but are not routinely tested for in clinical practice.24 Only one patient in our series had an established genetic cancer syndrome involving p53. Telomere shortening is associated with treatment-related myelodysplastic syndrome/AML in lymphoma patients after autologous stem cell transplant; however, this has not been studied in the nontransplantation, long-term survivor population.25
The main limitation of this analysis is the small number of late subsequent leukemias, which precludes identification of definitive associations with therapeutic exposures. However, the continued follow-up of the large and aging CCSS cohort and the extensive confirmatory process using central review to validate these cases allows identification of this novel finding. Therapy received in this historic cohort may differ slightly from modern therapies; however, alkylating agents, anthracyclines, and radiation therapy remain the backbone of treatment for a considerable proportion of pediatric cancers. Another limitation includes the absence of confirmed treatment information for the subsequent leukemias, making interpretation of the survival probability in these cases more difficult.
This is the first description of increased risk of subsequent leukemia ≥ 15 years from primary malignancy, demonstrating a 3.5-fold increased risk above that of the general population. This challenges current screening practices put forth by the Children's Oncology Group long-term follow-up guidelines, which recommend a screening complete blood count up to 10 years after diagnosis. A high level of suspicion should be maintained for long-term survivors presenting with pancytopenia, particularly those exposed to radiation and/or anthracycline and alkylating agent therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (grant U24-CA55727; L.L.R.). Support to St Jude Children's Research Hospital was also provided by the Cancer Center Support (grant CA 21765) and the American Lebanese-Syrian Associated Charities.
National Institutes of Health
Authorship
Contribution: K.N., J.L., J.P.N., S.B., S.H., W.L., A.M., D.S., L.L.R., and G.T.A. conceived and designed the study; K.N., S.H., Z.L., W.L., and D.S. analyzed and interpreted data; K.N. and G.T.A. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregory T. Armstrong, St Jude Children's Research Hospital, 262 Danny Thomas Pl, MS 735, Memphis, TN 38105; e-mail: greg.armstrong@stjude.org.