Abstract
Antiphospholipid syndrome is characterized by thrombosis and/or recurrent pregnancy loss in the presence of antiphospholipid antibodies (APLAs). The majority of APLAs are directed against phospholipid-binding proteins, particularly β2-glycoprotein I (β2GPI). Anti-β2GPI antibodies activate endothelial cells in a β2GPI-dependent manner through a pathway that involves NF-κB. Krüppel-like factors (KLFs) play a critical role in regulating the endothelial response to inflammatory stimuli. We hypothesized that activation of endothelial cells by APLA/anti-β2GPI antibodies might be associated with decreased expression of KLFs, which in turn might facilitate cellular activation mediated through NF-κB. Our experimental results confirmed this hypothesis, demonstrating markedly decreased expression of KLF2 and KLF4 after incubation of cells with APLA/anti-β2GPI antibodies. Restoration of KLF2 or KLF4 levels inhibited NF-κB transcriptional activity and blocked APLA/anti-β2GPI–mediated endothelial activation despite NF-κB p65 phosphorylation. Chromatin immunoprecipitation analysis demonstrated that inhibition of NF-κB transcriptional activity by KLFs reflects sequestration of the cotranscriptional activator CBP/p300, making this cofactor unavailable to NF-κB. These findings suggest that the endothelial response to APLA/anti-β2GPI antibodies reflects competition between KLFs and NF-κB for their common cofactor, CBP/p300. Taken together, these observations are the first to implicate the KLFs as novel participants in the endothelial proinflammatory response to APLA/anti-β2GPI antibodies.
Introduction
The antiphospholipid syndrome (APS) is characterized by arterial or venous thrombosis and/or recurrent fetal loss in the presence of antiphospholipid antibodies (APLAs).1-3 It is now widely accepted that the majority of pathologic antibodies in patients with this disorder are actually directed against phospholipid-binding proteins, the most common of which is β2-glycoprotein I (β2GPI).
The pathogenesis of APS-associated thrombosis is multifactorial, and a number of mechanisms have been proposed.4 These include inhibition of protein C activation and activity,5,6 inhibition of annexin V assembly on exposed phospholipid surfaces,7 and prevention of appropriate interactions of antithrombin with glycosaminoglycans,8 among others.4,9 Studies from our laboratory and others suggest that β2GPI-dependent activation of vascular cells by APLA/anti-β2GPI antibodies plays a central role in disease pathogenesis10,11 and may initiate the cascade of events that leads to thrombus development. For example, β2GPI binds to endothelial cell annexin A2, and subsequent cross-linking of annexin A2-bound β2GPI initiates endothelial cell activation through a pathway that may involve Toll-like receptor 4 (TLR-4)11-13 and leads to activation of NF-κB.14 A similar pathway may be functional in monocytes, activation of which also contributes to the development of thrombosis in patients with APLA.15 In addition, APLA may promote platelet activation in the presence of subthreshold concentrations of agonists,16 although whether this is a receptor-mediated process, and if so, its relationship to platelet β2GPI binding sites, such as GP1b17 and apoER2,18 requires further study.
In endothelial cells, activation of NF-κB stimulates an inflammatory and procoagulant response19 and plays a critical role in the ability of APLA to promote thrombosis.20 Thus, modulation of NF-κB activity may provide an opportunity to reverse the pathologic vascular response in APS. The Krüppel-like factors (KLFs),21 particularly KLF2 and KLF4, inhibit inflammatory cytokine-mediated responses in endothelial cells,22,23 at least in part through inhibition of NF-κB activity.23 However, expression of KLF2 itself may be inhibited by inflammatory cytokines and/or vascular injury,23-25 although the expression of KLF4 appears to be increased under these conditions.26
In considering the importance of NF-κB in endothelial cell activation mediated by APLA/anti-β2GPI antibodies14 and the potentially opposing effects of KLF2 and KLF4, we hypothesized that changes in expression of these transcription factors might influence the endothelial cell response to APLA/anti-β2GPI antibodies. Here, we report that, unlike responses to inflammatory cytokines, the expression of both KLF2 and KLF4 is decreased in response to APLA/anti-β2GPI antibodies. Moreover, restoring the expression of these KLFs blocks endothelial cell activation in response to APLA/anti-β2GPI antibodies. These activities result from inhibition of NF-κB transcriptional activity through sequestration of the transcriptional coactivator CBP/p300 by KLFs.
Methods
Materials
All reagents were purchased from Sigma-Aldrich unless otherwise specified. Expression plasmids containing KLF2 and KLF4 open reading frames have been previously described.23,27 An expression plasmid containing the CBP/p300 open reading frame was a kind gift from Dr Andreas Hecht (Institut für Molekulare Medizin und Zellforschung). An NF-κB luciferase reporter construct was obtained from Stratagene, and an E-selectin luciferase reporter was a kind gift from Dr Paul DiCorleto (Cleveland Clinic Foundation). A dominant negative NF-κB plasmid construct (IκB super-repressor mutant) was a kind gift from Dr Nywana Sizemore (Cleveland Clinic Foundation). Goat anti–human E-selectin antibodies (C20) and antibodies against the NF-κB p65 subunit were from Santa Cruz Biotechnology. Antibodies against NF-κB phospho-p65 serine 536 were from Cell Signaling Technologies. Antibodies against human KLF4 were from CeMines. Antibodies against β-actin were from Abcam. Control rabbit immunoglobulin G (IgG) and murine IgG1 (MOPC-21) were from Zymed Laboratories. The peroxidase substrate Turbo-TMB was obtained from Pierce-Thermo Scientific. DNAse I and all quantitative PCR primers were obtained from Invitrogen. Reverse transcriptase buffer, RNAse inhibitor, and Moloney murine leukemia virus reverse transcriptase were obtained from New England Biolabs. SYBR Green Master Mix and 96-well quantitative PCR reaction plates were from Applied Biosystems. Polyvinylidene fluoride (PVDF) membranes (Immobilon-P) were from Millipore. ECL-Plus reagent was obtained from Perkin-Elmer, and autoradiographic film was from Kodak.
Anti-β2GPI antibodies from rabbits immunized with β2GPI were affinity purified using a column of Affigel HZ to which purified human β2GPI was coupled (Bio-Rad).11 Antibodies from an APS patient with high titers of anti-β2GPI antibodies (> 70 GPL units) and recurrent deep venous thrombosis were affinity-purified in an identical manner.
Cell culture and reagents
HUVECs were isolated as previously described.28 Cells were maintained in Medium 199 containing 10% FBS, penicillin-streptomycin, and endothelial cell growth supplement, isolated as described by Maciag et al.29 Cells were maintained at 37°C in a humidified atmosphere of 10% CO2 and 90% air. All experiments were performed using HUVECs of passage 3 or lower.
Purification of β2GPI
β2GPI was isolated as described previously.12 Briefly, outdated, fresh frozen plasma was subjected to precipitation with polyethylene glycol. The precipitate was collected by centrifugation, and β2GPI was subsequently isolated by sequential chromatography on heparin-ultraflow (Sterogene) and S-Sepharose (GE Healthcare). In a final step, the isolated protein was run through a Detoxigel column to remove contaminating endotoxin. Purified β2GPI migrated as a single band of approximately 50 kDa under nonreducing conditions, with an apparent increase to approximately 62 kDa after reduction, and was recognized on immunoblotting by anti-β2GPI antibodies.
Immunoblotting
For immunoblotting studies, endothelial cells grown to confluence on 6-cm2 plates were treated with test reagents and washed with ice-cold PBS before preparation of cell extracts in radioimmunoprecipitation assay buffer (Tris 50mM, 150mM NaCl, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1% NP-40) containing 1mM phenylmethylsulfonyl fluoride and a 1:100 dilution of protease inhibitor cocktail. Alternatively, protein was precipitated from cell extracts prepared using Trizol (for RNA isolation). Briefly, after isolation of RNA and ethanol precipitation of DNA, proteins were precipitated from the remaining phenol-containing supernatant with acetone, and the precipitate resuspended in radioimmunoprecipitation assay buffer. Protein concentrations were determined using the Bio-Rad protein assay, with bovine serum albumin as standard. Equal amounts of protein (10-100 μg) from cells treated under different experimental conditions were separated on a 12% SDS-PAGE gel, and then transferred to a PVDF membrane. Membranes were blocked overnight using 3% BSA in PBS and then blotted with primary antibodies against NF-κB p65, phospho-p65 serine 536, β-actin, or KLF4. Bound primary antibody was detected using species-specific horseradish peroxidase-conjugated secondary antibodies at a concentration of 1:2000 to 1:5000. Bands were visualized using ECL-Plus reagent and exposure to autoradiography film.
Assessment of endothelial cell activation
Endothelial cell activation in response to APLA/anti-β2GPI antibodies was assessed primarily by the use of a previously described ELISA for measurement of E-selectin expression on the endothelial cell surface.11 Briefly, confluent monolayers of endothelial cells in 96-well microplates were incubated with test materials for 4 hours and then washed and fixed using 0.1% glutaraldehyde. Cells were then blocked with PBS containing 5% nonfat milk, washed with Tris-buffered saline containing 0.01% Tween 20, and incubated with 1 μg/mL goat anti–human E-selectin. Bound antibodies were detected using a 1:6000 dilution of horseradish peroxidase-conjugated rabbit anti–goat IgG, followed by the peroxidase substrate, Turbo-TMB. Relative amounts of endothelial cell bound E-selectin antibodies were determined by measuring A450.
In selected experiments, we also measured the effect of β2GPI/anti-β2GPI antibodies on E-selectin transcriptional activity. Briefly, endothelial cells were cotransfected with 0.5 μg of plasmid containing the E-selectin promoter coupled to luciferase and an identical amount of Renilla-luciferase plasmid (as a control for transfection efficiency). Forty-two hours after transfection, cells were incubated for 5 hours with medium alone, β2GPI (100nM), and rabbit anti–human β2GPI antibodies (600nM), or TNF-α (10 ng/mL). After treatment, cells were washed, lysed with Reporter Lysis Buffer (Luciferase Assay System Kit; Promega), and analyzed for Firefly and Renilla luciferase activities using the dual-Luciferase Reporter Assay system (Promega) and a luminometer (Victor3 Multilabel Counter; PerkinElmer Life and Analytical Sciences). Luciferase reporter activity was normalized by dividing Firefly luciferase activity by Renilla luciferase activity for each sample.
Measurement of KLF2, KLF4, and E-selectin expression by quantitative real-time PCR
Quantitative real-time PCR was used to assess levels of KLF2 and/or KLF4 after incubation of endothelial cells with β2GPI and/or anti-β2GPI antibodies, using a previously described protocol.26 Briefly, total RNA was isolated using Trizol. A total of 1 μg of total RNA was treated with DNAse I (Invitrogen) for 15 minutes at room temperature, after which the reaction was stopped via addition of 2.5mM EDTA and heating at 65 degrees for 10 minutes. RNA was reverse-transcribed using reverse-transcriptase buffer containing MgCl2 (2.2mM), 2.0mM 2′-deoxynucleoside 5′-triphosphates, 0.2 units/μL RNase inhibitor, and 0.5mM oligo-dT primers (Invitrogen) in 20-μL volume reactions. Annealing of oligo-dT was accomplished by incubating reactions for 2 minutes at 70°C. Samples were then placed on ice, after which 0.3 units/μL Moloney murine leukemia virus reverse transcriptase was added and cDNA prepared using a 2-step cycle consisting of 48°C for 1 hour and 94°C for 5 minutes. cDNA was then diluted to 80 μL for quantitative PCR analyses.
Quantitative PCR was performed using an ABI 7500 real-time PCR machine. Each reaction contained 5 μL SYBR Green Master Mix, 0.2μM forward primer, 0.2μM reverse primer, and 2-μL diluted cDNA from the previous step, in a final volume of 10 μL (primers used for quantitative PCR analyses are listed in Table 1). The following parameters were used for quantitative PCR: denaturation at 94°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 62°C for 60 seconds. GAPDH was used as an internal control. Fold induction was calculated after normalization to GAPDH using the ΔΔCt method.30 Dissociation curves showed a single amplification product in each reaction. All data represent at least 4 experiments, with each data point performed in triplicate.
Effects of KLF2, KLF4, and CBP/p300 on cellular activation
To assess the effects of KLF2, KLF4, and CBP/p300 on the response of endothelial cells to APLA/anti-β2GPI antibodies, we modulated the cellular levels of these factors by transfecting endothelial cells with expression vectors for the KLFs. Briefly, cells were plated in 12-well plates and allowed to achieve confluence. KLF2 or KLF4 expression was induced through transient transfection using Lipofectamine 2000. Briefly, each well was incubated with plasmid DNA for 3 hours (in experiments where transfection with more than one plasmid was performed simultaneously, scrambled DNA was used to assure that each well received the same amount of total DNA), after which the DNA/transfection reagent mixture was replaced with fresh serum-free medium. Cells were then incubated for 24 hours, washed with PBS, replated in 96-well plates, and incubated overnight at 37°C. Eighteen hours after replating (42 hours after transfection), cells were incubated for 5 hours with either medium alone, β2GPI (100nM) and rabbit anti–human β2GPI antibodies (600nM), or TNF-α (10 ng/mL). Cells were then analyzed for cell surface E-selectin expression.
In selected experiments, we used a CBP/p300 expression plasmid or siRNA to CBP/p300 to determine whether manipulation of intracellular levels of CBP/p300 modulated the cellular response to APLA/anti-β2GPI antibodies in the absence or presence of plasmid-mediated expression of KLF2 or KLF4. Briefly, endothelial cells were transfected simultaneously with nontargeting siRNA (scrRNA, control) or specific siRNA to CBP/p300, or a CBP/p300 expression plasmid in the absence or presence of expression plasmids for KLFs, and an NF-κB–luciferase reporter plasmid. For these studies, plasmid and siRNA were introduced into cells via electroporation using the HUVEC Nucleofector kit and Nucleofector transfection device (Lonza Walkersville) and cultured for 36 hours before exposure to test reagents and measurement of luciferase activity.
Assessment of NF-κB phosphorylation, nuclear translocation, and transcriptional activity
Phosphorylation, nuclear translocation, and transcriptional activity of NF-κB were assessed using complementary approaches. NF-κB phosphorylation and nuclear translocation were assessed in cytoplasmic and nuclear extracts by immunoblotting with antibodies specific for p65 serine 536. Nuclear or cytoplasmic extracts were prepared as previously described.31 Briefly, extracts were first prepared in a buffer containing 10mM Tris-HCl, pH 8.0, 60mM KCl, 1mM ethylenediaminetetraacetic acid, 1mM dithiothreitol, protease inhibitors, and 0.1% NP-40, then centrifuged at 750g at 4°C for 5 minutes, and the supernatant saved as the cytoplasmic extract. The nuclear pellet was resuspended in a buffer containing 20mM Tris-HCl (pH 8.0), 420mM NaCl, 1.5mM MgCl2, 0.2mM ethylenediaminetetraacetic acid, and 25% glycerol, and 5M NaCl was then added to reach a final NaCl concentration of 400mM. Samples were incubated for 10 minutes on ice, then vortexed and centrifuged at 15 000g for 4 minutes; the supernatant was saved as the nuclear extract.
To assess NF-κB transcriptional activity, endothelial cells were cotransfected with 0.5 μg of a plasmid containing the NFκB promoter coupled to luciferase and an identical amount of Renilla-luciferase plasmid. After exposure of cells to APLA/anti-β2GPI antibodies, NF-κB transcriptional activity was measured and normalized to Renilla luciferase as described for E-selectin.
ChIP–quantitative PCR assay
ChIP–quantitative PCR assays were performed as previously described.32 Endothelial cells were transfected with scrDNA or KLF expression vectors. Twenty-four hours later, media was removed and cells incubated for an additional 5 hours in medium containing either no additives (control) or β2GPI and anti-β2GPI antibodies. Cells were then treated with 1% formaldehyde for 15 minutes, and cell lysates were prepared and sheared by sonication. Lysates were precleared by incubation for 3 hours with protein G-Sepharose beads, using salmon sperm DNA and BSA as blocking agents. Equal amounts of chromatin were then immunoprecipitated using either an antibody to CBP/p300 (Abcam) or control rabbit IgG. After dissociation of protein from DNA, and purification of DNA using phenol-chloroform extraction, the purified DNA was used as a template for quantitative PCR amplification using primers that amplified all 3 NF-κB–binding sites on the E-selectin promoter (Table 1). As an internal control, primers for GAPDH were used to amplify corresponding sequences from the same templates. Reactions were performed in triplicate and data are presented as fold change over DNA input.
Statistical analysis
Data points are expressed as the mean ± SEM. All experimental points were measured in triplicate or quadruplicate, and all assays were repeated a minimum of 3 times. Differences between control and experimental conditions were assessed using the Student 2-tailed t test for paired samples. Statistical significance was defined as P < .05.
Results
β2GPI and anti-β2GPI antibodies activate endothelial cells
Previous studies from our laboratory and others have shown that anti-β2GPI antibodies activate endothelial cells in the presence of β2GPI.10,11 We confirmed that incubation of endothelial cells with β2GPI and anti-β2GPI antibodies induced endothelial cell activation, as determined by measurement of cell surface E-selectin expression. Both β2GPI and anti-β2GPI antibodies were required for activation because cells incubated with β2GPI or anti-β2GPI antibodies alone were not activated (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Similar results were seen when affinity-purified human anti-β2GPI antibodies from a patient with APS were incubated with endothelial cells in the presence of β2GPI (supplemental Figure 1B). Endothelial cell activation in both experiments was similar in magnitude to that which occurred in response to10 ng/mL TNF-α. The fact that endothelial cell activation occurred over a wide range of β2GPI and anti-β2GPI concentrations, with increased E-selectin expression at higher anti-β2GPI antibody concentrations (supplemental Figure 2) is consistent with activation occurring through a specific, receptor-mediated process.
Endothelial cell activation induced by APLA/anti-β2GPI antibodies results in decreased expression of KLF2 and KLF4
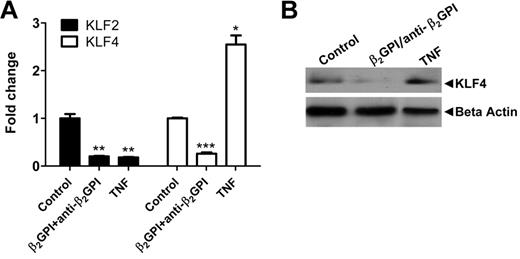
To examine the effects of APLA/anti-β2GPI antibody-induced endothelial cell activation on the expression of KLF2 and KLF4, we used quantitative PCR to measure the expression of mRNA encoding these transcription factors in endothelial cells exposed to β2GPI and anti-β2GPI antibodies. We observed a profound decrease in the expression of both KLF2 and KLF4 mRNA in endothelial cells treated in this manner (Figure 1A), which was maximal between 6 and 12 hours (supplemental Figure 3) after addition of β2GPI and anti-β2GPI antibodies to cells. These changes contrasted with those that occurred in response to TNF-α, which caused decreased KLF2 expression accompanied by increased expression of KLF4 (Figure 1A). Decreased expression of KLF4 by endothelial cells exposed to β2GPI and anti-β2GPI antibodies corresponded to decreased KLF4 protein (Figure 1B), although a lack of suitable antibodies to KLF2 precluded us from parallel analyses of KLF2 protein.
Endothelial cell activation induced by APLA/anti-β2GPI antibodies decreases expression of KLF2 and KLF4. (A) Cells were incubated with medium alone (control), β2GPI (100nM)/anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours. Total RNA was then isolated and KLF2 and KLF4 expression analyzed by quantitative real-time PCR. β2GPI/anti-β2GPI antibodies and TNF-α reduced KLF2 mRNA 5-fold compared with control (**P < .001 for both), whereas KLF4 mRNA levels were reduced 3.8-fold in the presence of β2GPI/anti-β2GPI antibodies (***P < .0001) but increased 2.6-fold by TNF-α (*P = .0012). Error bars represent the mean ± SEM of triplicate points. (B) Immunoblotting for KLF4 protein. Cells were treated as in panel A. Extracts were prepared and total protein (80 μg) separated using 7.5% SDS-PAGE, transferred to PVDF, and probed with goat anti–human KLF4 antibodies.
Endothelial cell activation induced by APLA/anti-β2GPI antibodies decreases expression of KLF2 and KLF4. (A) Cells were incubated with medium alone (control), β2GPI (100nM)/anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours. Total RNA was then isolated and KLF2 and KLF4 expression analyzed by quantitative real-time PCR. β2GPI/anti-β2GPI antibodies and TNF-α reduced KLF2 mRNA 5-fold compared with control (**P < .001 for both), whereas KLF4 mRNA levels were reduced 3.8-fold in the presence of β2GPI/anti-β2GPI antibodies (***P < .0001) but increased 2.6-fold by TNF-α (*P = .0012). Error bars represent the mean ± SEM of triplicate points. (B) Immunoblotting for KLF4 protein. Cells were treated as in panel A. Extracts were prepared and total protein (80 μg) separated using 7.5% SDS-PAGE, transferred to PVDF, and probed with goat anti–human KLF4 antibodies.
Restoration of KLF2 or KLF4 expression blocks APLA-mediated activation
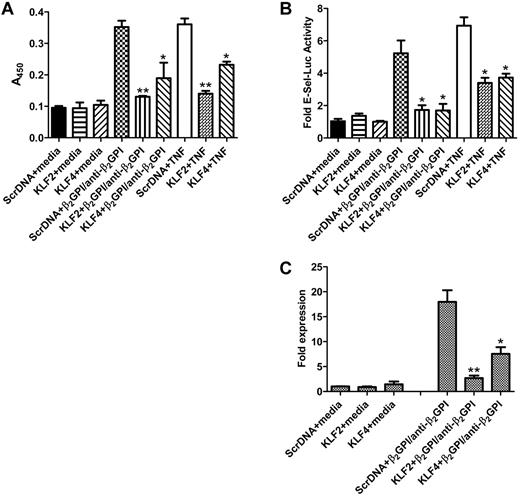
KLF2 and KLF4 preserve endothelial cell homeostasis by maintaining cells in a nonactivated state; thus, the decreased expression of these factors that occurs in response to APLA/anti-β2GPI antibodies would be expected to be permissive for cellular activation. To address this hypothesis, we determined whether restoration of KLF2 and KLF4 levels through plasmid-mediated expression blocked endothelial cell activation in response to APLA/anti-β2GPI antibodies. Endothelial cells were transfected with KLF2 and KLF4 expression plasmids and subsequently incubated in the presence of β2GPI and anti-β2GPI antibodies. Expression of either transcription factor blocked endothelial cell activation, as determined by a failure of transfected cells to augment cell surface E-selectin expression (Figure 2A). Inhibition of E-selectin expression occurred at the transcriptional level because, unlike cells transfected with a control vector, KLF2 or KLF4-transfected cells neither increased transcription of an E-selectin promoter-luciferase reporter construct (Figure 2B) nor increased production of E-selectin mRNA, as determined using quantitative PCR (Figure 2C). These results demonstrate that KLF2 and/or KLF4 maintain endothelial cells in a quiescent state in the presence of APLA/anti-β2GPI antibodies and suggest that decreased expression of these factors in response to such antibodies plays a permissive role in the endothelial cell activation response.
Restoration of KLF2 or KLF4 protects endothelial cells from APLA-mediated activation. (A) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control), or KLF2 or KLF4 cDNA. Transfected cells were then incubated with medium alone, β2GPI (100nM) and anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours, after which endothelial cell activation was measured using an E-selectin enzyme-linked immunosorbent assay. Activation was blocked in cells transfected with KLF2 or KLF4, but not scrambled DNA (**P < .01 and *P < 0.05 for KLF2 or KLF4, respectively, vs control). KLF2 and KLF 4 also blocked cellular activation in response to TNF-α. Error bars represent the mean ± SEM of quadruplicate points, and data are representative of 4 experiments. (B) Cells were treated as in panel A but cotransfected with an E-selectin-luciferase reporter and Renilla luciferase (to control for transfection efficiency). Endothelial cell activation was measured as a function of E-selectin luciferase activity normalized to Renilla. KLF2 and KLF4 inhibited E-selectin transcription compared with cells transfected with the control vector (*P < .02 for each) Error bars represent the mean ± SEM of triplicate points, and data are representative of 3 experiments. (C) Cells were transfected with KLF expression plasmids as described in panel A and incubated with medium alone (control) or β2GPI and anti–human β2GPI antibodies for 5 hours. Total RNA was then isolated, and E-selectin mRNA expression was analyzed by quantitative real-time PCR. Expression of KLF2 or KLF4 reduced the induction of E-selectin mRNA expression in response to β2GPI and anti-β2GPI antibodies by 6.7- and 2.6-fold, respectively (**P < .001 and *P < 0.01, respectively). Error bars represent the mean ± SEM of triplicate points.
Restoration of KLF2 or KLF4 protects endothelial cells from APLA-mediated activation. (A) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control), or KLF2 or KLF4 cDNA. Transfected cells were then incubated with medium alone, β2GPI (100nM) and anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours, after which endothelial cell activation was measured using an E-selectin enzyme-linked immunosorbent assay. Activation was blocked in cells transfected with KLF2 or KLF4, but not scrambled DNA (**P < .01 and *P < 0.05 for KLF2 or KLF4, respectively, vs control). KLF2 and KLF 4 also blocked cellular activation in response to TNF-α. Error bars represent the mean ± SEM of quadruplicate points, and data are representative of 4 experiments. (B) Cells were treated as in panel A but cotransfected with an E-selectin-luciferase reporter and Renilla luciferase (to control for transfection efficiency). Endothelial cell activation was measured as a function of E-selectin luciferase activity normalized to Renilla. KLF2 and KLF4 inhibited E-selectin transcription compared with cells transfected with the control vector (*P < .02 for each) Error bars represent the mean ± SEM of triplicate points, and data are representative of 3 experiments. (C) Cells were transfected with KLF expression plasmids as described in panel A and incubated with medium alone (control) or β2GPI and anti–human β2GPI antibodies for 5 hours. Total RNA was then isolated, and E-selectin mRNA expression was analyzed by quantitative real-time PCR. Expression of KLF2 or KLF4 reduced the induction of E-selectin mRNA expression in response to β2GPI and anti-β2GPI antibodies by 6.7- and 2.6-fold, respectively (**P < .001 and *P < 0.01, respectively). Error bars represent the mean ± SEM of triplicate points.
Expression of KLF2 or KLF4 inhibits NF-κB transcriptional activity in response to APLA/anti-β2GPI antibodies
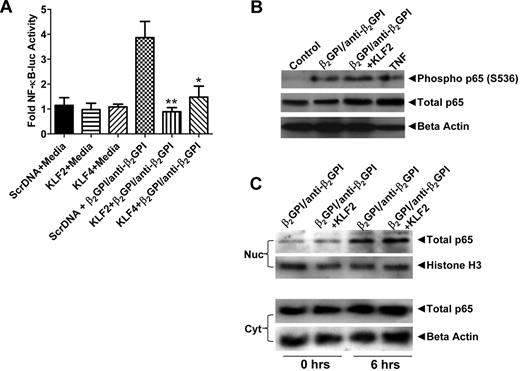
Previous studies have demonstrated that APLA/anti-β2GPI antibody-mediated cellular activation occurs, at least in part, through stimulation of NF-κB, leading to downstream responses that enhance cellular procoagulant and inflammatory activity.19 We confirmed that, in the system used in these studies, inhibition of NF-κB using a dominant negative NF-κB construct blocked endothelial cell activation as determined by transcription of E-selectin mRNA (supplemental Figure 4). To assess the mechanisms by which alterations in KLF2 or KLF4 expression might regulate responses to APLA/anti-β2GPI antibodies, we assessed the effects of altered levels of these factors on the phosphorylation of NF-κB p65, the nuclear translocation of phospho-p65, and NF-κB transcriptional activity. Plasmid-induced expression of either KLF2 or KLF4 potently inhibited the transcriptional activity of NF-κB in response to APLA/anti-β2GPI antibodies, as determined using an NF-κB promoter-luciferase reporter construct (Figure 3A). Intriguingly, however, expression of the KLF transcription factors blocked neither the phosphorylation of NF-κB p65 (Figure 3B) nor the translocation of phospho-p65 to the nucleus (Figure 3C). Indeed, the increase in phospho-p65 in nuclear extracts induced by APLA treatment was similar to that observed for TNF-α.
Expression of KLF2 or KLF4 inhibits NF-κB transcriptional activity in APLA/anti-β2GPI–treated endothelial cells. (A) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control), or KLF2 or KLF4 cDNA along with an NF-κB–luciferase reporter and Renilla luciferase (as a transfection efficiency control). Transfected cells were subsequently incubated with medium alone, β2GPI (100nM) and anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours before measurement of luciferase activity. NF-κB transcriptional activity was determined from measured NF-κB luciferase activity normalized to Renilla. Expression of KLF2 and KLF4 inhibited NF-κB transcriptional activity in the presence of β2GPI/anti-β2GPI antibodies compared with control cells (**P < .004 and *P < 0.04, respectively). Error bars represent the mean ± SEM of triplicate points, and data are representative of 4 experiments. (B) KLF2 expression does not inhibit phosphorylation of p65 serine 536. Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control), or KLF2 cDNA and subsequently treated as in panel A. Cell extracts were prepared, and 80 μg of total protein was separated using 7.5% SDS-PAGE, transferred to PVDF, and blotted with rabbit anti–human antibodies to phospho-p65 (serine 536) and total p65. (C) KLF2 expression does not block nuclear translocation of NF-κB p65. Endothelial cells were transfected and treated as in panel B. Nuclear (Nuc) and cytoplasmic (Cyt) extracts were prepared, and 40 μg of protein from each was separated by 7.5% SDS-PAGE, transferred to PVDF, and blotted with rabbit anti–human p65. Transfection of endothelial cells with a KLF4 expression vector yielded identical results as seen with KLF2 in panels B and C.
Expression of KLF2 or KLF4 inhibits NF-κB transcriptional activity in APLA/anti-β2GPI–treated endothelial cells. (A) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control), or KLF2 or KLF4 cDNA along with an NF-κB–luciferase reporter and Renilla luciferase (as a transfection efficiency control). Transfected cells were subsequently incubated with medium alone, β2GPI (100nM) and anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours before measurement of luciferase activity. NF-κB transcriptional activity was determined from measured NF-κB luciferase activity normalized to Renilla. Expression of KLF2 and KLF4 inhibited NF-κB transcriptional activity in the presence of β2GPI/anti-β2GPI antibodies compared with control cells (**P < .004 and *P < 0.04, respectively). Error bars represent the mean ± SEM of triplicate points, and data are representative of 4 experiments. (B) KLF2 expression does not inhibit phosphorylation of p65 serine 536. Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control), or KLF2 cDNA and subsequently treated as in panel A. Cell extracts were prepared, and 80 μg of total protein was separated using 7.5% SDS-PAGE, transferred to PVDF, and blotted with rabbit anti–human antibodies to phospho-p65 (serine 536) and total p65. (C) KLF2 expression does not block nuclear translocation of NF-κB p65. Endothelial cells were transfected and treated as in panel B. Nuclear (Nuc) and cytoplasmic (Cyt) extracts were prepared, and 40 μg of protein from each was separated by 7.5% SDS-PAGE, transferred to PVDF, and blotted with rabbit anti–human p65. Transfection of endothelial cells with a KLF4 expression vector yielded identical results as seen with KLF2 in panels B and C.
Inhibition of NF-κB activity in APLA/β2GPI-treated HUVECs by KLF2 involves CBP/p300
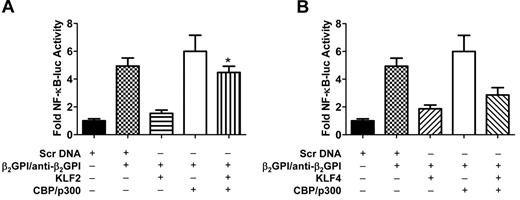
Previous studies have demonstrated that the transcriptional coactivator CBP/p300 may interact with and serve as a cofactor for both KLFs and NF-κB.24,33-35 This consideration, taken together with the results depicted in Figure 3, suggested that the ability of KLF2 and KLF4 to inhibit APLA/anti-β2GPI antibody-stimulated NF-κB transcriptional activity without impairing NF-κB p65 phosphorylation or nuclear translocation might reflect an indirect effect of the KLFs on NF-κB. To address this issue, we transfected endothelial cells with KLF2 or KLF4 in the absence or presence of a CBP/p300 expression vector; all cells were also cotransfected with an NF-κB luciferase reporter vector. Cells were then incubated with APLA/anti-β2GPI antibodies, and cellular activation assessed in parallel with NF-κB transcriptional activity. These studies demonstrated that expression of CBP/p300 partially restored NF-κB transcriptional activity in APLA/anti-β2GPI–treated endothelial cells that had been transfected with a KLF2 expression vector (Figure 4A). Similar results were observed in KLF4-transfected cells, although their magnitude was of borderline statistical significance (P = .056), suggesting that additional mechanisms may underlie the ability of KLF4 to inhibit endothelial activation by APLA/anti-β2GPI antibodies (Figure 4B).
Coexpression of CBP/p300 restores NF-κB transcriptional activity in APLA/anti-β2GPI–activated endothelial cells in the presence of KLF2 or KLF4. (A) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control) or KLF2, and/or CBP/p300. All cells were also transfected with an NF-κB–luciferase reporter and Renilla luciferase. Cells were subsequently treated with medium alone, β2GPI (100nM)/anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours before measurement of NF-κB luciferase activity, which was normalized to Renilla. KLF2 expression inhibited NF-κB transcriptional activity in the presence of β2GPI/anti-β2GPI antibodies, although inhibition was reversed by CBP/p300 (*P < .005) in the presence of CBP/p300 compared with KLF2 + β2GPI/anti-β2GPI alone. Error bars represent the mean ± SEM of triplicate points, and data are representative of 3 independent experiments. (B) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control) or KLF4, and/or CBP/p300. All cells were also transfected with an NF-κB–luciferase reporter and Renilla luciferase. KLF4 expression inhibited NF-κB transcriptional activity in the presence of β2GPI and anti-β2GPI antibodies; inhibition was partially reversed by CBP/p300 (P = .056). Error bars represent the mean ± SEM of triplicate points, and data are representative of 3 independent experiments.
Coexpression of CBP/p300 restores NF-κB transcriptional activity in APLA/anti-β2GPI–activated endothelial cells in the presence of KLF2 or KLF4. (A) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control) or KLF2, and/or CBP/p300. All cells were also transfected with an NF-κB–luciferase reporter and Renilla luciferase. Cells were subsequently treated with medium alone, β2GPI (100nM)/anti-β2GPI antibodies (600nM), or TNF-α (10 ng/mL) for 5 hours before measurement of NF-κB luciferase activity, which was normalized to Renilla. KLF2 expression inhibited NF-κB transcriptional activity in the presence of β2GPI/anti-β2GPI antibodies, although inhibition was reversed by CBP/p300 (*P < .005) in the presence of CBP/p300 compared with KLF2 + β2GPI/anti-β2GPI alone. Error bars represent the mean ± SEM of triplicate points, and data are representative of 3 independent experiments. (B) Endothelial cells were transfected with expression vectors containing scrambled DNA (Scr DNA, control) or KLF4, and/or CBP/p300. All cells were also transfected with an NF-κB–luciferase reporter and Renilla luciferase. KLF4 expression inhibited NF-κB transcriptional activity in the presence of β2GPI and anti-β2GPI antibodies; inhibition was partially reversed by CBP/p300 (P = .056). Error bars represent the mean ± SEM of triplicate points, and data are representative of 3 independent experiments.
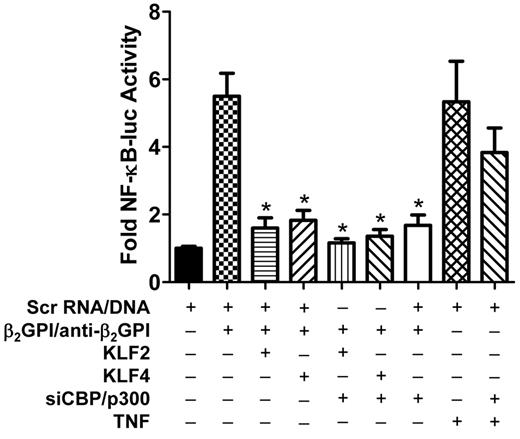
To examine the dependence of these effects on CBP/p300, we also determined the effects of siRNA-mediated knockdown of CBP/p300 on NF-κB activity in endothelial cells exposed to APLA/anti-β2GPI antibodies after transfection with KLF2 or KLF4 expression vectors. In these studies, cells were simultaneously transfected with siRNA to CBP/p300, and KLF2, KLF4, or scrambled DNA, and an NF-κB luciferase reporter. Cells were then incubated with β2GPI and anti-β2GPI antibodies, and luciferase activity was measured in cell lysates. We observed that siCBP/p300 diminished NF-κB activity in endothelial cells exposed to APLA/anti-β2GPI antibodies regardless of whether the cells had been cotransfected with KLF2 or KLF4 expression vectors (Figure 5). These results suggest that CBP/p300 plays an essential role in regulating the activation state of endothelial cells in response to APLA/anti-β2GPI antibodies, presumably by serving as a coactivator with NF-κB, and supports a model in which KLFs regulate the activity of NF-κB by competing with NF-κB for CBP/p300 cofactor activity. The fact that CBP/p300 knockdown did not block endothelial cell activation induced by TNF-α suggests a different activation mechanism by the latter.
Inhibition of NF-κB activity in APLA/anti-β2GPI–treated HUVECs by KLF2 or KLF4 is in part the result of CBP/p300. Endothelial cells were transfected with scrambled DNA (Scr DNA, control), or KLF2 or KLF4, in the absence or presence of siRNA to CBP/p300. All cells were also transfected with an NF-κB–luciferase reporter and Renilla luciferase. Cells were subsequently incubated with either medium alone, β2GPI (100nM), and anti–human β2GPI antibodies (600nM), or TNF (10 ng/mL) for 5 hours, then lysed before determination of NF-κB–dependent luciferase activity. KLF2 and KLF4 expression, in the absence of siCBP/p300, inhibited NF-κB transcriptional activation in the presence of β2GPI and anti-β2GPI antibodies compared with cells transfected with the control vector (*P < .008). siCBP/p300 inhibited NF-κB transcriptional activity independently, as well as in the presence of KLF2 or KLF4 (*P < .008). However, siCBP/p300 did not significantly affect NF-κB transcriptional activity in the presence of TNF-α. Error bars represent the mean ± SEM of quadruplicate points and data are representative of 3 independent experiments.
Inhibition of NF-κB activity in APLA/anti-β2GPI–treated HUVECs by KLF2 or KLF4 is in part the result of CBP/p300. Endothelial cells were transfected with scrambled DNA (Scr DNA, control), or KLF2 or KLF4, in the absence or presence of siRNA to CBP/p300. All cells were also transfected with an NF-κB–luciferase reporter and Renilla luciferase. Cells were subsequently incubated with either medium alone, β2GPI (100nM), and anti–human β2GPI antibodies (600nM), or TNF (10 ng/mL) for 5 hours, then lysed before determination of NF-κB–dependent luciferase activity. KLF2 and KLF4 expression, in the absence of siCBP/p300, inhibited NF-κB transcriptional activation in the presence of β2GPI and anti-β2GPI antibodies compared with cells transfected with the control vector (*P < .008). siCBP/p300 inhibited NF-κB transcriptional activity independently, as well as in the presence of KLF2 or KLF4 (*P < .008). However, siCBP/p300 did not significantly affect NF-κB transcriptional activity in the presence of TNF-α. Error bars represent the mean ± SEM of quadruplicate points and data are representative of 3 independent experiments.
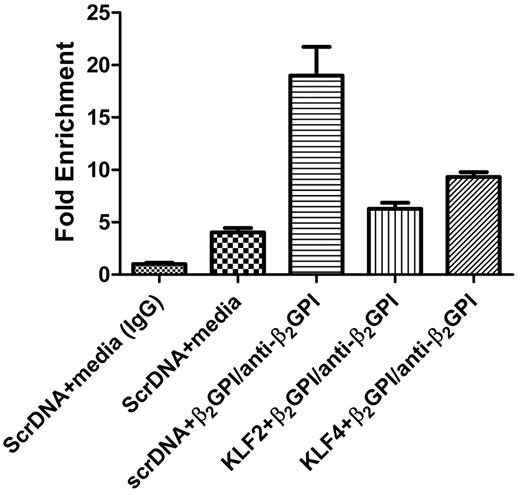
To further explore this proposed model, we performed a ChIP assay to determine whether plasmid-mediated expression of KLF2 or KLF4 in endothelial cells blocked the ability of CBP/p300 antibodies to immunoprecipitate DNA sequences encompassing NF-κB–binding sites in the E-selectin promoter after exposure of cells to β2GPI and anti-β2GPI antibodies. Briefly, cells were transfected with scrambled DNA or KLF2 or KLF4 expression vectors and then exposed to β2GPI and anti-β2GPI antibodies. After this incubation and the induction of protein-DNA cross-links, lysates were immunoprecipitated with anti-CBP/p300 antibodies, and DNA was isolated and amplified using primers specific for the NF-κB–binding sites in the E-selectin promoter. In cells transfected with scrambled DNA, immunoprecipitation with the CBP/p300 antibody coprecipitated DNA that was potently amplified by these primers. In contrast, amplification of DNA from CBP/p300 immunoprecipitates of KLF2- or KLF4-transfected cells yielded a signal that was only slightly increased above the baseline obtained from control cells (Figure 6). These studies are consistent with our hypothesis that KLF2 or KLF4 sequesters CBP/p300, blocking its interactions with NF-κB and thus its coactivator activity for NF-κB transcriptional targets (Figure 7).
ChIP analysis demonstrates that KLF2 and KLF4 sequester CBP/p300 and decrease CBP/p300-NF-κB complex formation and binding to NF-κB–binding sequences in the E-selectin promoter. Cells were transfected with scrambled DNA or KLF2 or KLF4 expression vectors and then incubated with medium alone or with β2GPI and anti-β2GPI antibodies for 5 hours. After formaldehyde treatment, cell lysates were immunoprecipitated with control IgG or anti-CBP/p300 antibodies, and DNA within the immunoprecipitates was isolated and amplified using primers specific for NF-κB–binding sites in the E-selectin promoter. In cells transfected with scrambled DNA, immunoprecipitation with the CBP/p300 antibody coprecipitated DNA that amplified strongly with these primers, suggesting formation of an NF-κB–CBP/p300 complex bound to NF-κB–binding sites in the E-selectin promoter. In contrast, amplification of DNA from CBP/p300 immunoprecipitates of KLF2- or KLF4-transfected cells yielded a signal only slightly increased above the baseline obtained from control cells, suggesting decreased NF-κB–CBP/p300 complex formation and decreased binding of this complex to NF-κB–binding sites in the E-selectin promoter. Control IgG did not immunoprecipitate a sequence that could be amplified. The abundance of each CBP/p300 coprecipitated NF-κB–binding sequence was calculated as fold change relative to the amount precipitated by control IgG.
ChIP analysis demonstrates that KLF2 and KLF4 sequester CBP/p300 and decrease CBP/p300-NF-κB complex formation and binding to NF-κB–binding sequences in the E-selectin promoter. Cells were transfected with scrambled DNA or KLF2 or KLF4 expression vectors and then incubated with medium alone or with β2GPI and anti-β2GPI antibodies for 5 hours. After formaldehyde treatment, cell lysates were immunoprecipitated with control IgG or anti-CBP/p300 antibodies, and DNA within the immunoprecipitates was isolated and amplified using primers specific for NF-κB–binding sites in the E-selectin promoter. In cells transfected with scrambled DNA, immunoprecipitation with the CBP/p300 antibody coprecipitated DNA that amplified strongly with these primers, suggesting formation of an NF-κB–CBP/p300 complex bound to NF-κB–binding sites in the E-selectin promoter. In contrast, amplification of DNA from CBP/p300 immunoprecipitates of KLF2- or KLF4-transfected cells yielded a signal only slightly increased above the baseline obtained from control cells, suggesting decreased NF-κB–CBP/p300 complex formation and decreased binding of this complex to NF-κB–binding sites in the E-selectin promoter. Control IgG did not immunoprecipitate a sequence that could be amplified. The abundance of each CBP/p300 coprecipitated NF-κB–binding sequence was calculated as fold change relative to the amount precipitated by control IgG.
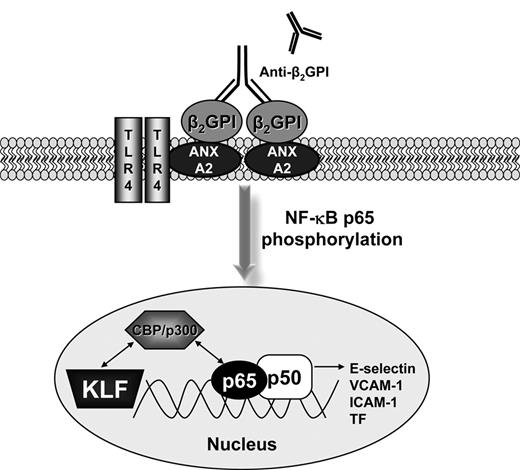
Model for KLF regulation of the activity of NF-κB in the presence of APLA/anti-β2GPI antibodies. CBP/p300 regulates the activation of endothelial cells because it is an essential cofactor for NF-κB and the KLFs. A dynamic equilibrium exists between binding of CBP/p300 to the KLFs and NF-κB. The decrease in KLF levels that occur during APLA/anti-β2GPI–induced endothelial cell activation allows the preferential association of CBP/p300 with NF-κB, thus promoting the transcription of NF-κB–dependent genes, such as cell surface adhesion molecules (E-selectin) and tissue factor.
Model for KLF regulation of the activity of NF-κB in the presence of APLA/anti-β2GPI antibodies. CBP/p300 regulates the activation of endothelial cells because it is an essential cofactor for NF-κB and the KLFs. A dynamic equilibrium exists between binding of CBP/p300 to the KLFs and NF-κB. The decrease in KLF levels that occur during APLA/anti-β2GPI–induced endothelial cell activation allows the preferential association of CBP/p300 with NF-κB, thus promoting the transcription of NF-κB–dependent genes, such as cell surface adhesion molecules (E-selectin) and tissue factor.
Discussion
The association of APLA/anti-β2GPI antibodies with thrombosis is widely appreciated, and recent studies have directly demonstrated that affinity-purified anti-β2GPI antibodies induce thrombosis in an animal model.36 However, the mechanisms underlying APLA/anti-β2GPI–mediated thrombosis remain incompletely understood. These antibodies inhibit key components of several naturally occurring anticoagulant pathways, including the thrombomodulin-mediated activation of protein C,37 the activity of activated protein C,28 and the assembly of the annexin V crystal shield on exposed phospholipid surfaces, among others.7 However, in order for such anticoagulant activities to be relevant, it is likely that a triggering event leading to activation of coagulation with attendant thrombin generation may be necessary. We hypothesize that this initial event involves, at least in part, the activation of endothelial cells, leading to increased expression of cell surface adhesion molecules that promote monocyte and platelet adhesion,11,38 the elaboration of microparticles,38-40 and the expression of tissue factor.41,42 Support for this hypothesis is supported by studies demonstrating that anti-β2GPI antibodies activate endothelial cells in a β2GPI-dependent manner11 and that elevated levels of endothelial cell and platelet-derived microparticles circulate in patients with APLA/anti-β2GPI antibodies, even in the absence of an acute thrombotic event.11,38,40,43 Defining the molecular pathways that facilitate vascular cell activation in patients with these antibodies may provide additional insight into disease pathogenesis and potentially suggest new directions for more specific therapeutic intervention than indefinite anticoagulation.
In this article we demonstrate that APLA/anti-β2GPI antibodies perturb a critical regulatory pathway that plays a central role in maintaining vascular homeostasis. Incubation of endothelial cells with APLA/anti-β2GPI antibodies led to profoundly decreased expression of KLF2 and KLF4, the primary KLFs present in endothelium.22 Interestingly, this response appears to be unique to APLA/anti-β2GPI antibodies, as inflammatory cytokines, such as IL-1 and TNF-α, decrease the expression of KLF2 but increase the expression of KLF4.25,26 Additional studies will be required to define the regulatory elements that underlie these diverse responses. Nevertheless, the dual decrease in expression of these factors in response to APLA/anti-β2GPI antibodies is probably a potent facilitator of endothelial cell activation.
We have also confirmed previous reports suggesting a key role for NF-κB in the response of endothelial cells to APLA/anti-β2GPI–induced activation19 and demonstrated that restoration of KLF2 and/or KLF4 levels blocked endothelial cell activation in response to these antibodies through an indirect effect on NF-κB transcriptional activity. In the presence of KLF2 or KLF4, NF-κB was activated normally, as determined by phosphorylation of serine 536 of the p65 subunit and translocation of p65 to the nucleus; however, the transcriptional activity of NF-κB was inhibited, as measured using an NF-κB promoter construct. Subsequent experiments demonstrated that this decrease in transcriptional activity by KLFs was the result of sequestration of CBP/p300, an essential transcriptional coactivator for NF-κB.24,35 Support for this mechanism is provided by the observation that decreasing the expression of CBP/p300 using specific siRNA completely blocked endothelial cell activation in response to APLA/anti-β2GPI antibodies, presumably by reducing the availability of CBP/p300 for binding NF-κB and enhancing its transcriptional activity. Finally, using ChIP, we demonstrated that immunoprecipitation of CBP/p300 protein led to coprecipitation of NF-κB–binding sites within the E-selectin promoter. However, plasmid-mediated expression of KLF2 or KLF4 prevented the coimmunoprecipitation of these sequences (Figure 6). Thus, we propose that a dynamic equilibrium exists between the KLFs, NF-κB, and CBP/p300 and that the state of this equilibrium regulates the susceptibility of endothelial cells to APLA/anti-β2GPI–induced activation. Indeed, we hypothesize that the decreased expression of KLFs that occurs in response to APLA/anti-β2GPI antibodies is essential for cellular activation, by freeing additional CBP/p300 for interactions with NF-κB. These findings also suggest that modulation of CBP/p300 activity might represent a potential pathway for desensitizing vascular endothelium to the effects of APLA/anti-β2GPI antibodies because reduction of CBP/p300 levels using specific siRNA blocked the ability of these antibodies to activate endothelial cells even when KLF levels were maintained through the use of expression vectors.
In endothelial cells, KLF2 stimulates the expression of numerous genes associated with endothelial quiescence and the maintenance of antithrombotic properties.23,24,44 Induction of thrombomodulin (THBD) and endothelial nitric oxide synthase (NOS), with decreased expression of PAI-1 (SERPINE 1) provide just a few examples of the more than 1000 genes whose expression is altered after KLF2 overexpression.23 Several studies have also demonstrated that statins, primarily fluvastatin, inhibit the expression of adhesion molecules45 and tissue factor23,46 on endothelial cells exposed to APLA, and in one report treatment of mice with fluvastatin inhibited the thrombogenic properties of APLA in the cremasteric muscle vasculature and femoral vein after mechanical injury.23,47 However, the molecular mechanisms underlying these effects were not defined. Recent work has demonstrated that several of the statins, including mevastatin, simvastatin, and lovastatin, are potent inducers of KLF2 in endothelial cells, and that the ability of statins to stimulate thrombomodulin and endothelial nitric oxide synthase expression is KLF2-dependent.48,49 The expression of KLF4 is also increased in the presence of simvastatin, lovastatin, and mevastatin,50,51 and this increase is dependent on ERK activation and confers vasoprotection.50 Taken together, these observations suggest that a potential mechanism by which statins may inhibit the thrombogenic properties of APLA/anti-β2GPI antibodies may involve inhibition of the ability of these antibodies to decrease the expression of KLFs, a situation that we hypothesize is essential for NF-κB transcriptional activity. Additional studies using in vitro and in vivo models will be needed to examine this hypothesis further.
In conclusion, our studies support a model in which APLA/anti-β2GPI–mediated inhibition of KLF expression facilitates activation of endothelial cells by promoting the deregulated activation and transcriptional activity of NF-κB and the expression of downstream prothrombotic, proinflammatory genes. These findings provide new insight into the molecular mechanisms of APLA/anti-β2GPI–mediated endothelial cell activation that may have therapeutic implications. Additional studies using in vivo approaches should aid in further defining these mechanisms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Daiji Kawanami for assistance with KLF2 and KLF4 transfection methodology.
This work was supported by the following grants from the National Institutes of Health: P50HL081011, K.R.M.; HL097593, HL086548, HL076754, HL084154, and HL075427, M.K.J.; and K08HL083090, A.H. K.L.A. was supported by T32HL007147 (to K.R.M.).
National Institutes of Health
Authorship
Contribution: K.L.A. designed the individual experiments, performed the primary data analysis, and wrote the manuscript; A.H. and M.K.J. provided guidance concerning the analysis of KLF2 and KLF4 data, provided KLF2 and KLF4 expression vectors, and edited the manuscript; and K.R.M. conceived the original hypothesis for this work and contributed to data analysis and editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Keith R. McCrae, Taussig Cancer Institute and Department of Cell Biology, R4-018, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: mccraek@ccf.org.