The etiology of familial thrombocytopenia can be difficult to determine, but novel mutations in the 5′UTR of ANKRD26 may represent a relatively common cause of autosomal dominant thrombocytopenia.
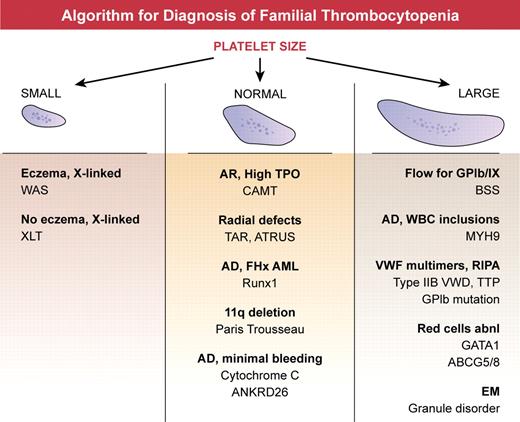
Inherited syndromes are relatively rare causes of thrombocytopenia, but elucidation of the genes underlying these disorders can provide valuable insights into mechanisms of megakaryopoiesis. In addition, some inherited syndromes predispose to the development of bone marrow failure or leukemia, and thus accurate diagnosis is important for clinical management and counseling. However, identification of the cause of thrombocytopenia in individual families can be difficult and genetic testing is not always readily available. A practical approach to diagnosis starts with evaluation of platelet size and morphology, refining the differential using apparent pattern of inheritance and associated features1 (see figure). For example, boys with thrombocytopenia and small platelets, with or without eczema and immune defects, should be evaluated for defects in the Wiskott Aldrich Syndrome protein (WASp) gene. Giant platelets may be seen in Bernard Soulier syndrome, MHY9-related disorder, Mediterranean macrothrombocytopenia because of ABCG5 or ABCG8 mutations, GATA1 mutations, and Gray platelet syndrome. Inheritance pattern, granule morphology, platelet function testing, and associated hematologic and nonhematologic features can help distinguish between these disorders. Normal-sized platelets are seen in congenital amegakaryocytic thrombocytopenia, thrombocytopenia with absent radii, amegakaryocytic thrombocytopenia with radioulnarsynostosis, Runx1 mutations, and mutation of cytochrome C (CYCS). Still, despite exhaustive evaluations, many families affected by thrombocytopenia cannot be diagnosed with a known disorder,1 suggesting that additional loci remain to be discovered.
Algorithm for diagnosis of familial thrombocytopenia. Platelet size, best determined by review of the peripheral smear, is a readily available measurement and forms the basis of the initial assessment. Small platelets are characteristic of mutations in WASp (Wiskott Aldrich Syndrome protein), located on the X chromosome. Large platelet disorders include BSS (Bernard Soulier syndrome), an autosomal recessive disorder with low to absent expression of platelet GPIb/IX complex; MYH9-related disorder, an autosomal dominant disorder with granulocyte inclusions and renal or hearing impairment; von Willebrand disease IIB, and mutation of GPIb, in which activating mutations in vW factor or platelet GPIb result in loss of high molecular weight vW factor multimers and enhanced platelet aggregation in response to low dose ristocetin; congenital thrombotic thrombocytopenic purpura (TTP) a recessive disorder with ultra-large vW factor multimers because of deficiency of ADAMTS13; GATA1 and ABCG5 or ABCG8 mutations, in which red cells are typically abnormal; and Gray platelet syndrome in which α granules are deficient. Normal-sized platelets are characteristic of congenital amegakaryocytic thrombocytopenia (CAMT), a recessive disorder that is associated with extremely high plasma thrombopoietin levels because of the absence of receptor mediated uptake; thrombocytopenia with absent radii (TAR) and amegakaryocytic thrombocytopenia with radioulnarsynostosis (ATRUS), which have characteristic skeletal malformations; Runx1 mutations, which result in autosomal dominant thrombocytopenia with predisposition to myeloid leukemias; Paris Trousseau syndrome, characterized by congenital heart defects and deletions of chromosome 11q23; autosomal dominant cytochrome C mutation; and the recently described mutations in the 5′UTR of ANKRD26 discussed here.
Algorithm for diagnosis of familial thrombocytopenia. Platelet size, best determined by review of the peripheral smear, is a readily available measurement and forms the basis of the initial assessment. Small platelets are characteristic of mutations in WASp (Wiskott Aldrich Syndrome protein), located on the X chromosome. Large platelet disorders include BSS (Bernard Soulier syndrome), an autosomal recessive disorder with low to absent expression of platelet GPIb/IX complex; MYH9-related disorder, an autosomal dominant disorder with granulocyte inclusions and renal or hearing impairment; von Willebrand disease IIB, and mutation of GPIb, in which activating mutations in vW factor or platelet GPIb result in loss of high molecular weight vW factor multimers and enhanced platelet aggregation in response to low dose ristocetin; congenital thrombotic thrombocytopenic purpura (TTP) a recessive disorder with ultra-large vW factor multimers because of deficiency of ADAMTS13; GATA1 and ABCG5 or ABCG8 mutations, in which red cells are typically abnormal; and Gray platelet syndrome in which α granules are deficient. Normal-sized platelets are characteristic of congenital amegakaryocytic thrombocytopenia (CAMT), a recessive disorder that is associated with extremely high plasma thrombopoietin levels because of the absence of receptor mediated uptake; thrombocytopenia with absent radii (TAR) and amegakaryocytic thrombocytopenia with radioulnarsynostosis (ATRUS), which have characteristic skeletal malformations; Runx1 mutations, which result in autosomal dominant thrombocytopenia with predisposition to myeloid leukemias; Paris Trousseau syndrome, characterized by congenital heart defects and deletions of chromosome 11q23; autosomal dominant cytochrome C mutation; and the recently described mutations in the 5′UTR of ANKRD26 discussed here.
In this issue of Blood, Noris and colleagues describe a novel type of mutation identified in their registry of patients with familial thrombocytopeniathat could not be diagnosed with an otherwise known disorder.2 Twenty-one of 210 families in their database had mutations in the 5′UTR region of ANKRD26, the ankyrin repeat domain 26 gene, on chromosome 10. The majority of these patients had normal-sized platelets, autosomal dominant inheritance, and relatively minor bleeding symptoms. More detailed investigation showed a deficiency of platelet GP1a and α granules, and where examined megakaryopoiesis was abnormal with increased numbers of dystrophic appearing megakaryocytes.
The function of ANKRD26 in megakaryocytes is not known, but the authors hypothesize that as a member of the POTE family of proapoptotic proteins, these 5-UTR mutations of ANKRD26 may lead to dysregulation of apoptosis.3 Apoptosis is thought to be mechanistically important not only in proplatelet formation and release, but also in regulating the circulating half-life of platelets. A recently described mutation of cytochrome C also enhances apoptosis and results in abnormal proplatelet formation, but affected patients had normal circulating platelet survival.4 POTE family proteins are up-regulated in several cancers,5 and thus it is intriguing that the authors in this study found an increased incidence of leukemia in their cohort of families with 5′UTR ANKRD26 mutations. Predisposition to myeloid leukemia is prominent in thrombocytopenia families with mutations of Runx1,6 because of haploinsufficiency of this stem cell transcription factor. It will be important to define the leukemia risk conferred by 5′UTR mutations in ANKRD26 to appropriately counsel patients with this disorder.
ANKRD26 would be the third gene on chromosome 10 to be implicated in autosomal dominant thrombocytopenia, the other 2 being acyl-coenzyme A binding domain containing protein 5 gene (ACBD5)7 and microtubule associated serine threonine like kinase (MASTL).8 In their previous study, the authors showed that a family reported to have a mutation of ACBD5, also carried a mutation in the 5′UTR of ANKRD26.3 Whether or not the family described with a mutation in MASTL also carries an ANKRD26 mutation is unknown, and no additional families with a mutation in MASTL have been identified. Given the apparent frequency of these mutations, evaluation for 5′UTR ANKDR26 mutation should be considered in the workup of patients with a family history of thrombocytopenia, especially if there is evidence for normal-sized platelets and autosomal dominant transmission.
The study by Noris et al shows the power of a well-characterized registry to study a rare condition such as inherited thrombocytopenia. With such a collaborative effort, a large cohort of patients can be analyzed not only to better understand known disorders but to define new genetic causes of thrombocytopenia that will lead to improved diagnostic testing and new avenues for research into thrombopoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■