Abstract
Peripheral blood stem cell transplantation (PBSCT) is the most common transplantation procedure performed in medicine. Its clinical introduction in 1986 replaced BM as a stem-cell source to approximately 100% in the autologous and to approximately 75% in the allogeneic transplantation setting. This historical overview provides a brief insight into the discovery of circulating hematopoietic stem cells in the early 1960s, the development of apheresis technology, the discovery of hematopoietic growth factors and small molecule CXCR4 antagonist for stem- cell mobilization, and in vivo experimental transplantation studies that eventually led to clinical PBSCT. Also mentioned are the controversies surrounding the engraftment potential of circulating stem cells before acceptance as a clinical modality. Clinical trials comparing the outcome of PBSCT with BM transplantation, registry data analyses, and the role of the National Marrow Donor Program (NMDP) in promoting unrelated blood stem-cell donation are addressed.
Introduction
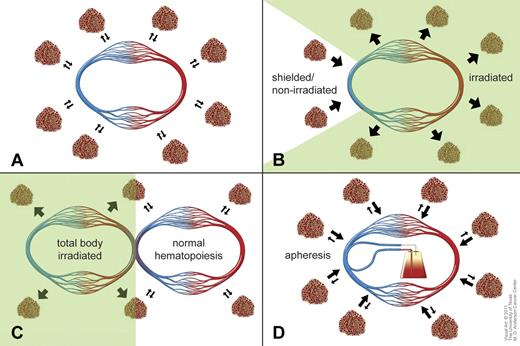
It has been 25 years since peripheral blood stem cell transplantation (PBSCT) was introduced as a transplant modality. This anniversary, therefore, marks the first clinical evidence that circulating hematopoietic stem cells can completely and permanently regenerate a lymphohematopoietic system after myeloablative treatment. Even more fundamental and beyond transplant aspects, the capability of hematopoietic blood stem cells to regenerate and maintain equal cellular concentration throughout all marrow spaces in the human body attributes to them a homeostatic function that had until then never been shown before in a clinical setting (Figure 1A).
Stem cell migration between BM and PB. (A) Stem cells are in dynamic equilibrium between PB and extravascular BM sites. Stem cell migration occurs via circulating blood. (B) Stem cell migration from nonirradiated BM via circulating blood to irradiated BM sites. (C) Stem cell migration through cross-circulation from a normal BM donor to a total body irradiated recipient. (D) Stem cell harvesting by apheresis. The apheresis-induced stem cell efflux from PB is compensated for by continuous stem cell influx from BM sites into circulating blood, thus preventing the circulating stem cell pool from becoming exhausted during apheresis.
Stem cell migration between BM and PB. (A) Stem cells are in dynamic equilibrium between PB and extravascular BM sites. Stem cell migration occurs via circulating blood. (B) Stem cell migration from nonirradiated BM via circulating blood to irradiated BM sites. (C) Stem cell migration through cross-circulation from a normal BM donor to a total body irradiated recipient. (D) Stem cell harvesting by apheresis. The apheresis-induced stem cell efflux from PB is compensated for by continuous stem cell influx from BM sites into circulating blood, thus preventing the circulating stem cell pool from becoming exhausted during apheresis.
Experimental evidence of circulating stem cells
The evolution of knowledge leading to PBSCT is quite remarkable. In the 1950s, the discovery of dividing, nonleukemic DNA-synthesizing cells in peripheral blood (PB) suggested the existence of a small number of circulating cells of “multipotential character.”1 Indirect evidence of the migration of stem cells via PB was first demonstrated in so-called shielding experiments, in which, after lethal total body irradiation, stem cells from shielded hematopoietic tissue areas entered the circulating blood and subsequently repopulated the irradiated BM (Figure 1B).2 Postradiation parabiosis experiments,3 followed later by cross-circulation experiments in large animals,4 provided clear evidence that circulating stem cells were capable of reconstituting an entire lymphohematopoietic system after myeloablative treatment (Figure 1C). The basic concept of blood stem-cell transplantation was formulated, a concept that can be seen as a repeat of embryonic hematopoietic development in which the vascularized cellular matrix becomes colonized through extravasation of circulating multipotent hematopoietic progenitors cells.5
The logical next step was to harvest white blood cells and, among them, putative stem cells to reconstitute lymphohematopoietic function in myeloablated BM (Figure 1D). In the early 1960s, the term “blood stem cell” as a transplantable cell population was introduced.6 BM rescue studies after autologous and allogeneic blood stem-cell transplantation into lethally irradiated large animals followed at Columbia University in Cooperstown, New York,7 and at the University of Washington School of Medicine in Seattle,8 but were not pursued at a clinical level because of the rarity of circulating stem cells at steady state compared with BM as a stem- cell source.
Development of clinical apheresis technology
The development of the continuous-flow apheresis technology at the National Institutes of Health (NIH) in the early 1960s and its first clinical application at The University of Texas M. D. Anderson Cancer Center in Houston was a critical next step. The design of the NCI-IBM blood cell separator allowed 2-3 times the patient's blood volume to be processed, resulting in large numbers of white blood cells to be collected in a matter of only 2-3 hours.9 It was, therefore, conceivable that large amounts of blood stem cells, a small subpopulation of white blood cells, could be harvested as well by use of the apheresis technology (Figure 1D). In the early 1970s, a first attempt at M. D. Anderson Cancer Center of collecting colony-forming stem cells by continuous-flow apheresis was successful,10 thus laying the foundation for clinical PBSCT as an equivalent to BMT, albeit apheresis-derived stem cell yield was still significantly inferior to that of multiple BM aspirations.
Failed clinical PBSCT attempts
In the mid-1970s, concerns were raised about the quality of circulating blood stem cells. They were identified as having a limited capacity for self-renewal and low proliferative potential. It was speculated that clonogenic blood-derived stem cells “… are victims of clonal senescence, and have been expelled as waste products from the bone marrow.”11 Two clinical blood stem-cell transplantation attempts among identical twins, one in 1979 at the University of California at Los Angeles12 and the other in 1980 at the NIH,13 resulted in failed or incomplete lymphohematopoietic reconstitution. In the University of California at Los Angeles case the leukapheresis product was administered to the twin patient as 6 separate white blood cell transfusions during the course of 14 days. The total amount of mononuclear cells transfused was 5.8 × 1010/kg recipient body weight. More than 2 months after transfusion, no platelet or granulocyte engraftment was documented. One may speculate that each individual white blood cell transfusion did not contain a sufficient amount of hematopoietic progenitor cells to enable engraftment. In the NIH case, the leukapheresis-derived mononuclear cells were administered as daily infusions during the course of 8 days. No recovery of granulocytes, monocytes, or platelets was documented at 8 weeks after transfusion. The authors attributed engraftment failure to the low number of clonogenic hematopoietic progenitor cells transfused being only one-eighth the quantity used in BM transplantation. Subsequently, both patients were rescued with their twin's marrow cells. It should be noted that, at that time, stem-cell collection by apheresis was performed at steady state without any growth factor priming.
First successful autologous PBSCTs
The autologous transplant modality required a liquid nitrogen cryopreservation technique for large volume leukapheresis products. Such a method was developed, allowing stem cells to accumulate over the course of several daily collections until an engraftment dose was reached.14
Thus, with the apheresis and cryopreservation technologies in place, blood stem-cell infusions were first attempted in 1981 at Hammersmith Hospital in London, United Kingdom, in patients with accelerated phase chronic myeloid leukemia (CML); the patients' stem cells had been harvested in earlier chronic phase with the intent of achieving a second chronic phase.15 In the same year, at Johns Hopkins Hospital in Baltimore, MD, a “normal” blood stem-cell harvest and transplantation attempt was undertaken in a patient with CML in cytogenetic remission. After myeloablative treatment and blood stem-cell infusion, there was first evidence of fast and complete hematopoietic reconstitution, although long-term stem-cell engraftment could not be documented.16
It was another 5 years, in 1986, before a patient with Burkitt lymphoma underwent myeloablative radio- and chemotherapy followed by PBSCT at Heidelberg University Hospital in Germany.17 Hematopoietic reconstitution was fast and complete. Twenty-five years later, the patient remains alive without evidence of disease and with normal lymphohematopoietic function.
In 1986 and 1987, similar reports of successful PBSCTs were documented by research groups at the University of Nebraska Medical Center,18 the Hospital Haut Leveque in Bordeaux, France,19 and the Royal Adelaide Hospital in Adelaide, Australia.20 In all of those initial PBSCT cases, stem cells were collected by multiple apheresis procedures at steady state without hematopoietic growth factor mobilization treatment.
Strategies to increase PBSC concentration
In contrast to the well-established BM transplantation, the use of PB stem cells in the mid-1980s seemed cumbersome mainly because in non-CML patients numerous steady-state apheresis procedures were required to achieve an engraftment stem-cell dose. For PBSCT to be accepted as a mainstream treatment modality, strategies to temporarily increase PB stem-cell concentration had to be developed. One strategy was to induce a rebound of PB stem-cell concentration after nonmyelotoxic chemotherapy and short-term myelosuppression.21 Another strategy was to temporarily expand the PB stem-cell pool by administering hematopoietic growth factors.
Hematopoietic growth factors and CXCR4 antagonist for optimizing stem-cell yield
Hematopoietic growth factors
Whereas the molecular cloning and clinical development of G-CSF initially focused on ameliorating the side effects of chemotherapy-induced cytopenia, it soon became obvious that G-CSF also expanded the circulating hematopoietic stem-cell pool by mobilizing CD34+38− hematopoietic progenitor cells from extravascular BM sites to PB.22 In 1988, 2 stem-cell mobilization studies in patients at the Dana-Farber Cancer Institute in Boston, MA,23 and at Royal Melbourne Hospital in Australia24 were published. Researchers at the Dana-Farber Cancer Institute used GM-CSF whereas G-CSF was used at the Royal Melbourne Hospital; these studies provided clear evidence of an increased PB concentration of colony-forming cells by 60-fold and up to 100-fold over baseline, respectively. Follow-up studies showed a mobilization advantage of G-CSF over GM-CSF,25 and today G-CSF is considered the gold standard of stem-cell mobilization treatment. By the mid-1990s, with those 2 stem-cell mobilization strategies (chemomobilization and/or G-CSF) in place, autologous PBSCT became increasingly accepted, eventually entirely replacing autologous BMT.
Healthy donors were initially excluded from donating G-CSF–mobilized hematopoietic stem cells primarily because of concerns about triggering lymphohematopoietic malignancies in genetically predisposed healthy sibling donors. Nevertheless, 5 years after the introduction of G-CSF as a stem-cell mobilization agent in patients, data of a first mobilization study on healthy donors were published by researchers at Sapporo Medical College in Japan.26 To ensure donor safety, initial guidelines for G-CSF-induced stem cell mobilization and collection in normal donors were formulated.27 A crucial next step was to open up PB stem-cell donation to unrelated donors. A prospective clinical study was initiated by the NMDP for G-CSF–mobilized stem-cell collections in second donation requests, followed in 1999 by an NMDP protocol for primary PB stem-cell collection in unrelated donors. Stem-cell collection data on 1488 G-CSF mobilized donors reported to the International Bone Marrow Transplant Registry and European Group for Blood and Marrow Transplantation (EBMT) revealed a comparable safety profile to BM harvesting.28 A 2008 conference report on clinical and ethical issues of using hematopoietic growth factors in healthy stem-cell donors29 gave a state-of-the-art insight into the safety of G-CSF mobilization treatment, and came to the following conclusions:
Severe but rare side effects of G-CSF mobilization treatment, including splenic rupture and sickle cell crisis, have been reported.
Data from the NMDP show an overall rate of 0.6% serious adverse events attributed to stem-cell mobilization treatment and collection.30
No definite conclusions can be made regarding the long-term effects of G-CSF treatment in healthy donors.
The balance between the risk to the donor and the benefit to the recipient raises ethical issues that are still being discussed.
According to the NMDP and its Be the Match Registry, 76% of adult unrelated donors, that is > 3100, donated G-CSF–mobilized PB stem cells. However, in pediatric patients BM and umbilical cord blood (UCB) are the predominant unrelated stem-cell sources.
Plerixafor
The interaction between the chemokine stromal cell-derived factor1 (SDF1, also known as CXCL12) and its receptor CXCR4 in BM plays a major role in the trafficking of progenitor cells between BM and PB.31 Whereas G-CSF induces stem-cell mobilization by down-regulating the expression of SDF1 on BM osteoblasts and by proteolytic cleavage of both SDF1 and CXCR4, plerixafor, a novel small molecule antagonist, reversibly inhibits the interaction of SDF1 with its cognate receptor CXCR4. With the safety of plerixafor in human volunteers documented,32 the first clinical study on the mobilization of hematopoietic progenitor cells in healthy donors was published in 2003 by researchers at the University of Washington in Seattle.33 A follow-up study in 2005 clearly showed a synergistic effect of G-CSF and plerixafor on the mobilization of CD34+ cells.34 Thus, the combination of G-CSF and plerixafor has become the standard treatment regimen in difficult to mobilize patients. In 2008, plerixafor was approved by the Food and Drug Administration for PB stem-cell mobilization in combination with G-CSF in patients with non-Hodgkin lymphoma and multiple myeloma.35 In 2009, 2 randomized phase 3 trials in patients with multiple myeloma36 and in patients with non-Hodgkin lymphoma37 confirmed those earlier studies.
The central role of the SDF1-CXCR4 axis in mobilizing hematopoietic stem cells also applies to the release of leukemic blast cells38 and progenitor cells of various lymphohematopoietic malignancies and solid tumors.39 As with G-CSF, there is concern about mobilizing clonogenic tumor cells when plerixafor is used. Long-term follow-up of patients transplanted with plerixafor-mobilized stem-cell autografts is needed to evaluate its safety.
Allogeneic PBSCT
Until the mid-1990s, allogeneic PBSCT was not considered a transplantation option due to its potential risk of inducing severe GVHD because of the 10-fold greater amount of donor T cells contained in a PB allograft. In addition, there was a hesitance to expose healthy stem-cell donors to cytokine treatment for stem cell mobilization.
In 1989, the first attempt to transplant nonmobilized, T-cell–depleted PB stem cells into a HLA-identical sibling at the University of Nebraska Medical Center in Omaha was made.40 There was early and exclusively donor-derived hematopoietic engraftment. Grade 2 acute GVHD was limited to the skin. Because the patient died of infectious complications 32 days after transplantation, long-term engraftment could not be demonstrated. Four years later, another single-case report on allogeneic stem-cell transplantation was published by researchers at Nottingham University Hospital in the United Kingdom.41 This time, G-CSF–mobilized stem cells were transplanted into an HLA-identical sibling. There was trilineage engraftment without GVHD. The follow-up, however, was only 58 days after transplantation and again lacked evidence of sustained engraftment.
In 1995, the first clinical trials of successful allogeneic PBSCTs at M. D. Anderson Cancer Center,42 Fred Hutchinson Cancer Research Center,43 and Kiel University Hospital in Germany,44 proved that concerns about inducing severe GVHD were wrong. The incidence of acute GVHD was surprisingly similar to that in patients who underwent allogeneic BMT, and hematopoietic growth factor exposure in healthy donors did not seem to impose an undue risk. Eventually, molecular evidence revealed that donor-derived multipotent stem cells transplanted into a conditioned recipient could permanently generate an entire lymphohematopoietic system. Indeed, at our institution the first patient with refractory acute myelogenous leukemia who received her brother's blood stem cells has a 100% donor-derived lymphohematopoietic system and has been in complete and sustained remission for 16 years. The allogeneic transplantation setting, therefore, provided definite clinical proof that hematopoietic progenitor cells mobilized into PB and harvested by apheresis can generate a complete and sustained lymphohematopoietic system.
UCB transplantation, an extension of PB stem cell harvesting into the newborn's cord blood, was first introduced in 1989 at Hôpital Saint-Louis in Paris, France,45 and has become a widely used stem-cell transplantation option in children and, to a more limited extent, in adults. For a detailed review of the historical events leading to the clinical introduction of UCB transplantation, and major follow-up studies, we refer to Eliane Gluckman's paper published in 2009.46
PB compared with BM as a source of hematopoietic stem cells
Clinical trials
The unexpected initial finding of similar acute GVHD rates after allogeneic BMT and PBSCT prompted the design of randomized prospective clinical trials. In 1998, the results of the first multicenter trial of the EBMT on 70 patients confirmed those early observations of similar incidence rates of moderate to severe acute GVHD and transplantation-related mortality between patients who underwent PBSCT and patients who underwent BMT.47 Randomized trials, either multicenter48 or single center,49 followed. The clinical findings included faster platelet, neutrophil and immune recovery, greater occurrence of chronic GVHD, and lower probability of relapse in patients after PBSCT. In 2001, a randomized multicenter trial50 on 172 patients that involved the Fred Hutchinson Cancer Research Center and the University of Washington Hospital in Seattle, the City of Hope Medical Center in Duarte, CA, and Stanford University Hospital in California came to the following conclusions:
Recovery of neutrophils and platelets was faster in patients who underwent PBSCT.
There was no significant difference between PBSCT and BMT in the cumulative incidence of grades II-IV acute GVHD at 100 days after transplantation.
There was no significant difference between PBSCT and BMT in the cumulative incidence of chronic GVHD at 2 years after transplantation, although a trend favoring less chronic GVHD after BMT was noticed.
Both the estimated overall probability of survival and the rate of disease-free survival at 2 years after transplantation were better in patients who underwent PBSCT. The benefit of greater survival rates was observed primarily in patients with more advanced malignancy.
Clinical findings of an EBMT-initiated prospective randomized trial51 that involved 350 patients with standard-risk leukemia turned out to have a somewhat different outcome: the incidence of grades II-IV acute GVHD was found to be significantly greater in patients who underwent PBSCT. There was a greater incidence of chronic GVHD after PBSCT. However, there were no significant differences in overall survival or leukemia-free survival between the BMT and PBSCT groups at the 3-year follow-up.
A large retrospective International Bone Marrow Transplant Registry and EBMT registry analysis52 that involved 824 patients revealed the following data: the incidence of chronic GVHD at 1 year was significantly greater in patients after PBSCT, and treatment-related mortality rates and leukemia-free survival rates were in favor of patients undergoing PBSCT in advanced stages of leukemia. A later retrospective EBMT registry analysis of 3465 adult patients with acute myeloid leukemia and acute lymphoblastic leukemia revealed again a greater incidence of chronic GVHD after PBSCT. However, rates of acute GVHD, leukemia-free survival and overall survival were similar after BMT and PBSCT.53
To test various clinical outcome parameters such as survival probability, engraftment characteristics, acute and chronic GVHD, relapse, and immunereconstitution in a more GVHD-prone transplantation setting, a phase 3, randomized, multicenter, prospective comparative study of G-CSF–mobilized PBSCT versus BMT with the use of HLA-compatible unrelated donors was designed. This trial was conducted under the auspices of the Blood and Marrow Transplant Clinical Trials Network. The study met its accrual target of 550 patients and closed enrollment in September 2009. At the time of this writing, patient outcome results have not been released.
PB versus BM allograft composition
The clinical outcome of stem-cell transplantation is in part determined by the cellular composition of the stem-cell graft. The number of G-CSF–mobilized CD34+ hematopoietic progenitor cells contained in PB stem-cell grafts is 3-fold greater than in BM grafts resulting in faster reconstitution of neutrophils and platelets after PBSCT as documented in most randomized trials and registry data analyses.
The approximately 10-fold greater number of CD3+ T cells contained in an unmanipulated PB stem-cell allograft did, against all predictions, not cause more severe or a greater incidence of acute GVHD. To explain these clinical findings, researchers at the Fred Hutchinson Cancer Research Center focused on the effects of G-CSF mobilization treatment on stem-cell products. Data were presented showing that, compared with BM allografts, the 50-fold greater number of CD14+ monocytes and monocyte progenitors contained in G-CSF–mobilized PB stem-cell allografts had a suppressive effect on donor T cells.54 G-CSF mobilization treatment was also found to have an immunomodulatory effect on the graft. By mobilizing preferential T helper 2-inducing dendritic cells, the cytokines IL-4 and IL-10 are produced, which are less inflammatory and, therefore, are associated with diminished GVHD-inducing ability.55
Cell subsets that are implicated in the GVL effect include CD4+ and CD8+ T cells, and natural killer cells. The significantly greater number of T cells contained in PB stem-cell allografts together with a 3-fold greater percentage of natural killer cells are believed to be associated with a more pronounced GVL effect, although clinical GVL data of various randomized trials, as mentioned previously, are not conclusive. For a current and detailed understanding of the mechanisms underlying GVHD and GVL effect, we refer readers to Shlomchik's56 and Bleakley and Ridell's reviews.57
Final remarks
According to the Center for International Blood and Marrow Transplant Research database, 98% of autologous stem-cell transplantations performed between 2002 and 2006 in patients who were older than 20 years were blood-derived. In the respective allogeneic transplantation setting, 74% were blood-derived, 24% BM-derived, and 2% UCB-derived. The circulating blood has therefore emerged as the predominant source of hematopoietic stem cells for grafting.
Organ transplantation, including BM aspiration, has been traditionally a domain of surgery. In contrast, PB and UCB stem cell harvesting and processing has become the responsibility of transfusion medicine and blood banking. Hematopoietic “organ” donation has been replaced by “blood” donation, lacking emotional, and, in some parts of the world, even religious limitations. Translational research played a major role in the clinical development of PBSCT, and the evolution of PBSCT, in turn, has made a remarkable contribution to our understanding of stem-cell biology.
Authorship
Contribution: M.K. wrote the manuscript; and E.F. provided archival material and documentation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Körbling, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas M. D. Anderson Cancer Center, Unit 423, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: mkorblin@mdanderson.org.