Abstract
POEMS syndrome is a rare clonal plasma cell disorder without standard treatment. Based on the efficacy and low toxicity of a combination of melphalan and dexamethasone (MDex) for light chain amyloidosis, we conducted a prospective study of MDex treatment for patients with newly diagnosed POEMS syndrome. Thirty-one patients (19 men) were enrolled and the median age at the time of diagnosis was 44 years (range, 32-68 years). All patients received 12 cycles of MDex treatment. Twenty-five patients (80.6%) achieved hematologic response including 12 (38.7%) complete remission and 13 (41.9%) partial remission. Of all 31 patients, the neurologic response rate was 100%, assessed by overall neuropathy limitation scale (ONLS). The initial neurologic response was observed in 24 patients (77.4%) at 3 months after treatment and the median time to maximal neurologic response was 12 months (range, 3-15 months). Moreover, MDex substantially improved the level of serum vascular endothelial growth factor and relieved organomegaly, extravascular volume overload, and pulmonary hypertension. Only 6 patients (19.3%) suffered from grade 3 adverse events during treatment. All patients are alive and free of neurologic relapse after the median follow-up time of 21 months. Therefore, MDex is an effective and well-tolerated treatment option for patients with newly diagnosed POEMS syndrome.
Introduction
POEMS syndrome is a rare form of plasma cell dyscrasia characterized by polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes.1 The pathogenesis of POEMS syndrome is not fully understood, but there is accumulating evidence suggesting that the high levels of serum vascular endothelial growth factor (VEGF) contributed to some specific features of POEMS syndrome such as extravascular volume overload, organomegaly, and hemangioma.2 Although the course of POEMS syndrome is chronic and the median survival is usually more than 5 years, the quality of life in these patients is very poor as a result of progressive peripheral neuropathy.3
There is no standard treatment for POEMS syndrome. Historically, local irradiation and low-dose alkylator along with steroids had been the mainstay of the treatment of POEMS syndrome.4 However, local irradiation is not indicated for patients with widespread osteosclerotic lesions or patients without bone lesions. Alkylator and corticosteroid regimen—for example, using melphalan and prednisone—has a low response rate and short duration of response, although it is less toxic.5 Recently, high-dose melphalan with autologous peripheral stem cell transplantation has made impressive progress in the treatment of POEMS syndrome; the response rate of neuropathy is almost 100% and the response rate of other specific features of POEMS syndrome is also 70%-90%. However, the treatment-related morbidity of transplantation is high, as 50% of patients had engraftment syndrome and 37% of patients required mechanical ventilation. In addition, the transplantation-related mortality is 7.4%, which is higher than the 2% mortality observed in multiple myeloma.6 Moreover, the cost of autologous transplantation is about 100 000 RMB (US $15 000) in China and not covered by insurance. This is expensive for many patients, whose annual income is < $1000. Lastly, transplantation is not indicated for elderly patients or for patients with serious organ failure. Therefore, there is an urgent need to develop an affordable and effective treatment with low toxicity for patients with POEMS syndrome in low-income countries.
Melphalan plus dexamethasone (MDex) is a common conventional chemotherapy for various plasma disorders such as multiple myeloma and light chain amyloidosis.7 Moreover, MDex is affordable and the cost of one cycle is less than 500 RMB (∼ $75) in China. Given that POEMS syndrome is similar to light chain amyloidosis in some clinical features (for example, the presence of M protein and neuropathy), we speculate that MDex can be an effective and affordable treatment for POEMS syndrome patients in China. Therefore, we conducted a single-center, prospective study to evaluate the efficacy and toxicity of the combination of melphalan and dexamethasone (MDex) for patients with newly diagnosed POEMS syndrome.
Methods
Study subjects
Forty consecutive patients with newly diagnosed POEMS syndrome were referred to Peking Union Medical College hospital from January 2008 to November 2009 who met the diagnosis criteria proposed by Dispenzieri et al.1 In brief, these consisted of 2 major criteria (polyneuropathy and monoclonal plasmaproliferative disorder) and 7 minor criteria (bone lesion, Castleman disease, organomegaly, edema, endocrinopathy, skin changes, and papilledema). Two major criteria and at least one minor criterion were required for a diagnosis. Of these 40 patients, 9 patients received high-dose melphalan with peripheral blood stem cell transplantation; the remaining 31 patients, who were unable to undergo transplantation for the following reasons—elderly (age > 65 years, n = 3), renal failure (serum creatinine > 1.5mg/dL, n = 4), pulmonary hypertension (systolic pulmonary arterial pressure > 50mmHg, n = 7), and financial reasons (unable to pay for the cost of transplantation, n = 17)—were enrolled to receive MDex. All patients gave written informed consent according to the study protocol, which was approved by the Peking Union Medical College hospital institutional review board, in accordance with the Declaration of Helsinki.
Treatment protocol
MDex consisted of oral melphalan (10 mg/m2 body surface area) plus oral dexamethasone (40 mg/d) on days 1 to 4 of every 28-day cycle. Prophylaxis with proton-pump inhibitor was administered on days −1 to 5. The planned number of cycles was 12 cycles unless grade 4 adverse events or progressive disease occurred. The dose of melphalan was adjusted according to renal function as follows: if the glomerular filtration rate (GFR) was < 60 mL/min, the dose of melphalan reduced to 7.5 mg/m2 body surface area; if the GFR was < 30 mL/min, the dose of melphalan reduced to 5 mg/m2 body surface area.
Response assessment
Hematologic response.
The assessments of M protein by serum and urine protein electrophoresis, serum and urine immunofixation electrophoresis, serum-free light chain assay, and quantitation of light chain in 24-hour urine samples were performed every 3 months. A hematologic response was defined according to the response criteria cited by light chain amyloidosis.8 Briefly, a complete hematologic response (CR) was defined as the complete disappearance of the monoclonal immunoglobulin or light chain in a serum or urine specimen; a partial hematologic response (PR) was defined as more than 50% reduction in these proteins; a progressive disease (PD) was defined as more than 50% increase in these proteins or the reappearance of the proteins after CR; and a stable disease (SD) included all the status other than CR, PR, and PD.
Neurologic response.
The overall neuropathy limitation scale (ONLS) was used to assess neurologic disability.9 The ONLS consists of an arm score and a leg score. The arm score ranges from 0 (normal) to 5 (disability in both arms preventing all purposeful movement) and the leg score ranges from 0 (normal) to 7 (restricted to wheelchair or bed most of the day, unable to make any purposeful movements of the legs). Therefore, the range of ONLS score was from 0 (no disability) to 12 (maximal disability). The evaluation of ONLS was conducted every 3 months. A neurologic response was defined as scale score reduction of at least 1. When we designed the study, we had planned to use the Neuropathy Total Symptom Score-6 (NTSS-6) scale to evaluate the sensory abilities.10 However, we found this scale very difficult to implement and abandoned it after evaluating the first 10 patients because this scale was very subjective and the results were unreliable.
None of the patients received ankle-foot orthotics or other supporting devices during evaluation.
Responses of other abnormalities.
Complete physical examination and computed tomography of chest and abdomen or ultrasound of abdomen were performed every 3 months to assess organomegaly (lymphadenopathy, splenomegaly, and hepatomegaly) and extravascular volume overload (edema, ascites, pericardial and pleural effusion). Responses of organomegaly and extravascular volume overload were defined as a complete relief of organomegaly and extravascular volume overload, respectively. Echocardiography was used to examine the systolic pulmonary arterial pressure (sPAP) of patients with pulmonary hypertension every 3 months. Normalization of sPAP (less than 40 mmHg) was considered as a response of pulmonary hypertension. Serum VEGF levels were measured by an enzyme-linked immunosorbent assay every 3 months as described elsewhere.11 Normal serum VEGF was less than 600 pg/mL, which was determined by measurements in 20 healthy persons.
All patients received the following further examinations at the time of diagnosis: lumbar puncture to evaluate cerebrospinal fluid; funduscopic test to reveal papilledema; and x-ray of skull, vertebrae (cervical, thoracic, and lumber vertebrae), pelvis, and bilateral femurs and humerus for complete bone survey.
Adverse events
Toxicity or adverse events during chemotherapy were documented and classified by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0.
Results
Patient characteristics
Thirty-one patients (19 men and 12 women) were enrolled in this study. The median age at diagnosis was 44 years (range, 32-68 years). The median time from symptoms to diagnosis was 19 months (range, 4-80 months). Twenty-seven patients (87.1%) had all 5 typical clinical features of POEMS syndrome. All patients had motor-sensor neuropathy and the median ONLS score was 4 (range, 1-10). Eight patients (25.9%) had mild neuropathy with an ONLS score of 1 or 2. All patients had detectable M protein by serum immunofixation electrophoresis and 3 (9.7%) patients had measurable hematologic disease (defined as serum M protein > 5 g/L or 24-hour urine M protein > 100 mg); the type of M protein was restricted to λ light chain including IgA-λ (74.2%) and IgG-λ (25.8%). Extravascular volume overload (edema, ascites, pleural and pericardial effusion), papilledema, thrombocytosis, and pulmonary arterial hypertension were also common in these patients. Table 1 outlines the main clinical features of these 31 patients at diagnosis.
All patients had finished 12 cycles of chemotherapy as planned and terminated the treatment afterward. The median time from diagnosis to treatment was 10 days (range, 1-34 days). The median time from initial treatment to the last follow-up visit was 21 months (range, 15-36 months).
Neurologic response
Of 31 patients, all (100%) responded to treatment for their neuropathy, and ONLS scores reduced by at least 1. The median ONLS score of 31 patients declined from 4 (range, 1-10) before treatment to 1 (range, 0-4) at the last visit. The initial responses were observed in 24 patients (77.4%) at 3 months after treatment and the median time to maximal response was 12 months. Moreover, 9, 7, 6, 8, and 1 patients had ONLS scores of 0, 1, 2, 3, and 4, respectively, at 15 months after treatment. A total of 70.9% of patients had an ONLS score no higher than 2. Table 2 shows the change in ONLS scores in 31 patients after treatment. We compared the neurologic responses of patients who were unable to receive transplants because of financial reasons and patients with medical reasons. There was no difference in the median ONLS score reduction between these 2 groups (3 vs 3).
Hematologic response
Twelve of 31 patients (38.7%) achieved hematologic CR, 13 patients (41.9%) achieved hematologic PR, and the remaining patients (n = 6, 19.4%) remained at SD status. The overall response rate (CR + PR) was 80.6%. However, neurologic response was not affected by the status of hematologic response. The median ONLS score reductions were 2 (range, 1-6) in nonhematologic responders (n = 6) and 3 (range, 1-7) in hematologic responders.
Responses of organomegaly, extravascular volume overload, and pulmonary hypertension
In 28 patients with organomegaly, 18 (64.3%) responded to chemotherapy; their lymphadenopathy, hepatomegaly, and splenomegaly resolved. Twenty of 28 patients (71.4%) complicated by extravascular volume overload responded to treatment. Among 15 patients with pulmonary hypertension (median sPAP 49 mmHg; range, 41-100 mmHg), the sPAP declined to normal levels (< 40 mmHg) in 14 patients (93.3%) after treatment.
Response of serum VEGF
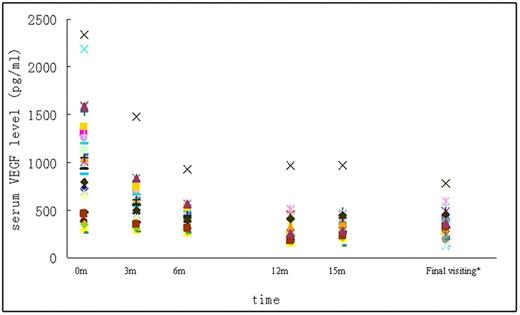
The median serum VEGF level was 1047 pg/mL (range, 249-2197 pg/mL) before treatment in all 31 patients, among whom high serum VEGF level was observed in 24 patients (77.4%). MDex treatment dramatically decreased the serum VEGF level. At the last follow-up visit, the median serum VEGF level was 312 pg/mL (range, 120-782 pg/mL). Normalization of serum VEGF level was observed in 23 (95.8%) of 24 patients; only 1 patient had an abnormal serum VEGF level at 15 months after treatment. Nonetheless, this patient's ONLS score declined from 5 to 3. Figure 1 shows the changes in serum VEGF level after treatment.
Changes in serum VEGF level in patients with POEMS syndrome after treatment. *The median time from final visit to initial treatment was 21 months.
Changes in serum VEGF level in patients with POEMS syndrome after treatment. *The median time from final visit to initial treatment was 21 months.
Adverse events
No treatment-related deaths were observed in this study. Grade 3 adverse events were uncommon, including 2 cases of neutropenia, 2 cases of thrombocytopenia, and 2 cases of bacterial pneumonia. No grade 4 adverse event was documented. The dose of melphalan was reduced in 2 patients because of grade 3 neutropenia. No patient discontinued therapy because of drug-related adverse events. Table 3 describes all adverse events of 31 patients during chemotherapy.
Follow-up
The median follow-up time in 31 patients was 21 months (range, 15-36 months) after the start of the therapy. All patients were alive and free of relapse during follow-up. No patients developed secondary myelodysplastic syndrome or acute leukemia.
Discussion
Our study is the first prospective study of treatment for patients with newly diagnosed POEMS syndrome and the first study using ONLS prospectively to assess the neurologic condition of POEMS syndrome. The ONLS is a concise and straightforward scale that is easy to follow up in an outpatient setting. It is recommended as an outcome measurement in clinical trials for various peripheral neuropathies.9 Our data show that the combination of melphalan and dexamethasone is very effective in POEMS syndrome patients, with a perfect neurologic response rate (100%) and a short time to initial neurologic response (77.4% response rate at 3 months after treatment and 12 months of time to maximal response). Although 8 of 31 patients have mild neuropathy with a very low ONLS score (0 or 1), the median ONLS score of all patients is 4, and patients with severe neuropathy (ONLS score ≥ 5) are not uncommon. Our results are superior to previous results reported in melphalan-based treatment, which yielded a neurologic response rate of 67% in 6 patients and a very long time to response (3-14 months).5 Our results are compared with those provided by high-dose melphalan with transplantation in previous retrospective studies. In 16 patients with POEMS syndrome who received high-dose melphalan (140 mg/m2 or 200 mg/m2) with transplantation at the Mayo Clinic, the neurologic response in 1 year was 87% and time to response was 3-6 months after transplantation.12 A 100% neurologic response was reported in 9 patients treated by transplantation in Japan and the neurologic improvements were observed at 1 to 3 months after treatment.13 Moreover, our study suggests that MDex is less toxic than transplantation. No death occurred and only 4 patients (12.9%) developed grade 3 adverse events during chemotherapy in the present study. Previously, 1 death occurred and 5 patients had respiratory failure during transplantation in the Mayo Clinic study, and 1 patient suffered from respiratory failure without treatment-related mortality during transplantation in Japan.
Our data show that melphalan plus dexamethasone can achieve a high hematologic response rate with 29.1% CR and 48.1% PR in patients with newly diagnosed POEMS syndrome. Interestingly, hematologic responders and nonresponders show no difference in neurologic improvement. The mechanism is yet unknown. We speculate that M protein may not be the sole etiological factor for neuropathy in POEMS syndrome, and cytokines including VEGF and/or others may partially contribute to neuropathy. Improvement of cytokine level induced by MDex may promote the reversal of neuropathy. Thus neurologic response rather than hematologic response is more desirable in the current treatment of POEMS syndrome. But more patients and longer follow-up time are needed to assess the relationship between hematologic response and relapse-free survival and/or overall survival. In addition to high neurologic response and hematologic response, our study further reveals that MDex can effectively relieve the main symptoms related to POEMS syndrome. The response rates of organomegaly and extravascular volume overload are 64.3% and 71.4%, respectively, which are comparable to previously reported results with transplantation (60%-100%).12,13 Pulmonary hypertension is a common clinical feature in POEMS syndrome. The incidence rate in 25 patients who received an echocardiogram in the first 2 years after diagnosis is 48% in the Mayo Clinic study.14 Few studies reported the treatment and outcome of POEMS syndrome–related pulmonary hypertension.15 In 15 patients with pulmonary hypertension in our study, the pulmonary arterial pressures in 14 patients (93.3%) returned to normal level after MDex treatment. Finally, melphalan with dexamethasone can significantly reduce the serum VEGF level, which has been considered one characteristic and a serum marker in POEMS syndrome and correlated with the activity of disease.16
The current study shows that MDex is very well tolerated with a low incidence rate of grade 3 adverse events and no grade 4 adverse events, which might be attributed partially to a younger population who were financially unable to undergo transplantation. That all patients are alive and free of neuropathy progression after the median follow-up time of 21 months shows that MDex can create a durable neurologic response. It is worth noting that the cumulative dose of melphalan is 480 mg/m2 in the MDex regimen and that the duration of exposure (total 48 days) of melphalan is much longer than that with transplantation (1 day). The risk of melphalan-related myelodysplasia or acute leukemia may increase with longer follow-up time, especially in young patients. Therefore, longer follow-up is needed to assess overall survival, progression-free survival, and long-term complications related to chemotherapy.
Finally, in this prospective study, we enrolled only patients with newly diagnosed POEMS syndrome who were unable to undergo transplantation in our study, because improved response and survival of transplantation had been reported by some previous retrospective studies and transplantation had been considered as the first-line treatment for POEMS syndrome in our center. However, patients in our study indeed consisted of 2 subgroups: one included 14 patients (45.2%) who were ineligible for transplantation because of elderly age or organ dysfunction, and the other included 17 low-income patients (54.8%) who could not afford but were otherwise eligible for transplantation. These 2 groups achieved similar improvement of neuropathy after treatment. Therefore, our results can be qualitatively considered representative of all patients with newly diagnosed POEMS syndrome regardless of eligibility for transplantation.
In conclusion, our study shows that combination of melphalan and dexamethasone is effective and well tolerated in patients with newly diagnosed POEMS syndrome. Obviously, more evidence, especially evidence from large-scale clinical trials, is needed to entertain the proposal that MDex may replace transplantation. However, it is fairly difficult to conduct a large clinical trial because of the rarity of this disease. International cooperation is highly desired for designing a randomized trial comparing conventional chemotherapy and autologous transplantation in POEMS syndrome.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Wei Su, Ms Wang Xuan, and Ms Lian-Feng Chen for technical assistance, and Dr Hui-chun Zhan and Dr Yu-lin Wang for helpful discussions.
Authorship
Contribution: J.L., H.-Z.G., Z.T., W.Z., and D.-B.Z. designed the study; J.L. and D.-B.Z., performed the study, analyzed data, and wrote the paper; H.-Z.G., W.Z., and Z.T. performed the study and analyzed data; M.-H.D., L.J., and W.G.Z. organized collection of patients samples; and all authors approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dao-Bin Zhou, Department of Hematology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, People's Republic of China; e-mail: daobinzhou@yahoo.com.